
 |
|||||

| Tracks 1-20 | ||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 1-2
![]() DR LOVE: What was reported at this year’s ASCO meeting related to
RCC that doctors in practice need to know about?
DR LOVE: What was reported at this year’s ASCO meeting related to
RCC that doctors in practice need to know about?
![]() DR HUDES: One important advance presented at this year’s ASCO meeting
was a trial conducted specifically for the patient who has experienced disease progression on sorafenib or sunitinib, the two approved vascular endothelial
growth factor (VEGF) tyrosine kinase inhibitors.
DR HUDES: One important advance presented at this year’s ASCO meeting
was a trial conducted specifically for the patient who has experienced disease progression on sorafenib or sunitinib, the two approved vascular endothelial
growth factor (VEGF) tyrosine kinase inhibitors.
What to do in the second line has been a conundrum. We’ve been moving from one approved drug to the next to the next, without conclusive data on what we’re accomplishing. In medicine we sometimes operate without data because we have to do the best we can for the patient. It was refreshing at this year’s ASCO meeting to hear of a trial that evaluated a drug — RAD001, also called everolimus — to find out how it would perform in the second-line setting (Motzer 2008).
![]() DR LOVE: What do we know about RAD001 as opposed to temsirolimus?
DR LOVE: What do we know about RAD001 as opposed to temsirolimus?
![]() DR HUDES: RAD001 is, like temsirolimus, an inhibitor of a mammalian
target of rapamycin — mTOR for short. This is an interesting target in
kidney cancer. It seems to control proliferation and angiogenesis. Four mTOR
inhibitors are now in existence. The parent drug, rapamycin, is only used
for prophylaxis of renal allograft rejection because of its immunosuppressive
properties. Temsirolimus, everolimus and another drug, deforolimus, are all
what we call rapalogs, or analogs of sirolimus or rapamycin.
DR HUDES: RAD001 is, like temsirolimus, an inhibitor of a mammalian
target of rapamycin — mTOR for short. This is an interesting target in
kidney cancer. It seems to control proliferation and angiogenesis. Four mTOR
inhibitors are now in existence. The parent drug, rapamycin, is only used
for prophylaxis of renal allograft rejection because of its immunosuppressive
properties. Temsirolimus, everolimus and another drug, deforolimus, are all
what we call rapalogs, or analogs of sirolimus or rapamycin.
Two of these agents have been tested in renal cancer. Temsirolimus, which was evaluated in the global ARCC study, is approved for kidney cancer. The ARCC trial was designed for patients with multiple adverse risk factors for short survival and showed a survival advantage with temsirolimus (Hudes 2007).
Later, the everolimus trial evaluated a different population — patients who had already received sunitinib or sorafenib. Patients were randomly assigned to treatment with everolimus or placebo, both with best supportive care. The study allowed a crossover to everolimus for patients who experienced disease progression on placebo.
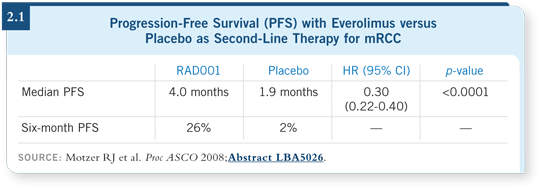
They saw a striking benefit from everolimus compared to placebo. The median progression-free survival was four months for everolimus and 1.9 months for placebo (Motzer 2008; [2.1]).
The activity of everolimus is not surprising. The fact that patients on placebo experienced disease progression in only 1.9 months, on one hand, is perhaps a little faster than we thought. On the other hand, we had data from an older randomized discontinuation study that showed that patients experienced disease progression quickly, in approximately two months, when they stopped sorafenib (Ratain 2004). So maybe these findings aren’t surprising after all.
![]() DR LOVE: What about response rates?
DR LOVE: What about response rates?
![]() DR HUDES: The response rate was low — about one percent for everolimus
and the same for placebo. The benefit is in stabilization of disease.
DR HUDES: The response rate was low — about one percent for everolimus
and the same for placebo. The benefit is in stabilization of disease.
![]() DR LOVE: When you view the data, do you believe there’s something there?
Two months doesn’t sound like much.
DR LOVE: When you view the data, do you believe there’s something there?
Two months doesn’t sound like much.
![]() DR HUDES: Yes — this is not a major lengthening of disease control, but it
does establish efficacy in terms of the median effect. Some patients on that
survival curve fare considerably better with disease control. As with a lot of
therapies, what we accomplish is incremental.
DR HUDES: Yes — this is not a major lengthening of disease control, but it
does establish efficacy in terms of the median effect. Some patients on that
survival curve fare considerably better with disease control. As with a lot of
therapies, what we accomplish is incremental.
Track 3
![]() DR LOVE: What about clinical issues with temsirolimus versus everolimus?
DR LOVE: What about clinical issues with temsirolimus versus everolimus?
![]() DR HUDES: The mTOR inhibitors are more alike than they are different.
The mechanism is specific for the mTOR protein kinase. Some differences
exist in formulation. Temsirolimus is only in an IV formulation now, whereas
everolimus is only in an oral formulation. In terms of the actual activity,
however, it would be difficult to prove that one is better than the other.
DR HUDES: The mTOR inhibitors are more alike than they are different.
The mechanism is specific for the mTOR protein kinase. Some differences
exist in formulation. Temsirolimus is only in an IV formulation now, whereas
everolimus is only in an oral formulation. In terms of the actual activity,
however, it would be difficult to prove that one is better than the other.
![]() DR LOVE: What about side effects and toxicity?
DR LOVE: What about side effects and toxicity?
![]() DR HUDES: Quite similar. Stomatitis is probably the most common side
effect. Some fatigue, anemia, rash and diarrhea occur — after stomatitis, these
are the most common toxicities. Pulmonary toxicity, which can occur in a few
patients — approximately eight percent in the everolimus study — can require
dose modification or temporary halting of treatment.
DR HUDES: Quite similar. Stomatitis is probably the most common side
effect. Some fatigue, anemia, rash and diarrhea occur — after stomatitis, these
are the most common toxicities. Pulmonary toxicity, which can occur in a few
patients — approximately eight percent in the everolimus study — can require
dose modification or temporary halting of treatment.
![]() DR LOVE: The specific pulmonary syndrome is interstitial pneumonitis.
DR LOVE: The specific pulmonary syndrome is interstitial pneumonitis.
![]() DR HUDES: Yes. It can be anything from ground glass infiltrates in an asymptomatic
patient, which is most common, to symptomatic dyspnea with bilateral
infiltrates, which require halting the treatment and treating with steroids. It’s
almost always reversible, however.
DR HUDES: Yes. It can be anything from ground glass infiltrates in an asymptomatic
patient, which is most common, to symptomatic dyspnea with bilateral
infiltrates, which require halting the treatment and treating with steroids. It’s
almost always reversible, however.
![]() DR LOVE: How do you generally utilize these agents?
DR LOVE: How do you generally utilize these agents?
![]() DR HUDES: For the patients with poor prognoses, I use temsirolimus in the
first line. I also consider it as second-line therapy for patients who have experienced
disease progression on sunitinib. For some patients, oral therapy is still
preferable. For some of these patients, I use sorafenib as second-line therapy.
DR HUDES: For the patients with poor prognoses, I use temsirolimus in the
first line. I also consider it as second-line therapy for patients who have experienced
disease progression on sunitinib. For some patients, oral therapy is still
preferable. For some of these patients, I use sorafenib as second-line therapy.
So many new agents are being tested now in kidney cancer that a practicing oncologist probably doesn’t have to look far for a second-line or even a third-line trial of a new tyrosine kinase inhibitor.
Track 10
![]() DR LOVE: Can you discuss what we know about combined biologic
therapy?
DR LOVE: Can you discuss what we know about combined biologic
therapy?
![]() DR HUDES: Combination therapy should be examined carefully. The BeST
trial (ECOG-E2804) is comparing single-agent bevacizumab to three combinations:
temsirolimus/bevacizumab, sorafenib/bevacizumab and temsirolimus/
sorafenib. At the time that this study was designed, the sunitinib combination
data were nonexistent.
DR HUDES: Combination therapy should be examined carefully. The BeST
trial (ECOG-E2804) is comparing single-agent bevacizumab to three combinations:
temsirolimus/bevacizumab, sorafenib/bevacizumab and temsirolimus/
sorafenib. At the time that this study was designed, the sunitinib combination
data were nonexistent.
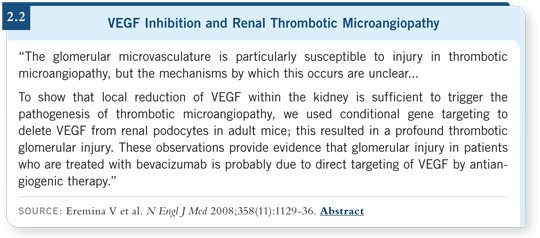
A study of sunitinib/bevacizumab was presented at ASCO by the group at Memorial Sloan-Kettering (Feldman 2008). Apropos to the need for safety data before proceeding, an unacceptable rate of microangiopathic thrombocytopenia and hemolytic syndrome occurred with long-term sunitinib/bevacizumab therapy.
It was not an anticipated toxicity. Microangiopathic syndrome is not common, but it is seen occasionally with single-agent bevacizumab (Eremina 2008; [2.2]).
![]() DR LOVE: What is the mechanism for this toxicity?
DR LOVE: What is the mechanism for this toxicity?
![]() DR HUDES: Direct damage to the endothelial cell, apparently affecting the
kidney. That’s one explanation for the occasional decline in renal function.
DR HUDES: Direct damage to the endothelial cell, apparently affecting the
kidney. That’s one explanation for the occasional decline in renal function.
![]() DR LOVE: Did they have enough data to evaluate efficacy in the sunitinib/
bevacizumab trial?
DR LOVE: Did they have enough data to evaluate efficacy in the sunitinib/
bevacizumab trial?
![]() DR HUDES: They did, and it wasn’t immediately clear that the response rate
or duration of response was better than you would expect with single-agent
sunitinib.
DR HUDES: They did, and it wasn’t immediately clear that the response rate
or duration of response was better than you would expect with single-agent
sunitinib.
The BeST study (2.3) is an important trial in terms of showing that combinations are feasible. Where do you set the bar for combination therapy to justify the anticipated extra toxicity of two drugs versus one drug? The alternative way of using multiple agents — in sequence — should be explored also.

Editor
Neil Love, MD
Interviews
Robert A Figlin, MD
- Select publications
Gary R Hudes, MD
- Select publications
Ronald M Bukowski, MD
- Select publications

| Terms of Use and General Disclaimer | Privacy Policy Copyright © 2008 Research To Practice. All Rights Reserved. |