
 |
|||||

| Tracks 1-22 | ||||||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Can you comment on the survival data from the trial of
sunitinib versus interferon as first-line therapy for mRCC?
DR LOVE: Can you comment on the survival data from the trial of
sunitinib versus interferon as first-line therapy for mRCC?
![]() DR FIGLIN: This is the trial that has changed the standard treatment for kidney
cancer. The study enrolled 750 patients and compared sunitinib to interferon.
DR FIGLIN: This is the trial that has changed the standard treatment for kidney
cancer. The study enrolled 750 patients and compared sunitinib to interferon.
It demonstrated more than a twofold prolongation in progression-free survival, and the objective response rate, both complete and partial, was almost 40 percent with sunitinib (Motzer 2007). Approximately 80 percent of patients experienced disease control, and in January 2006 sunitinib was approved for the treatment of mRCC.
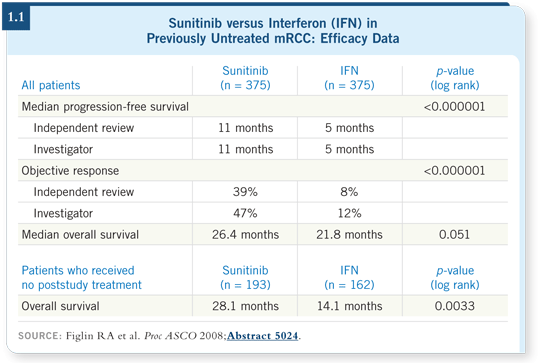
At ASCO in 2008 I presented the survival analysis, which showed that survival for the patients treated with sunitinib was 26.4 months compared to approximately 21 months in the control group (Figlin 2008; [1.1]).
The median survival for patients with metastatic kidney cancer in the interferon era was approximately 13 months, and this trial was designed to improve survival by 37.5 percent, increasing it to 17 months.
One might ask why the control group had a survival of 21 months, which is eight months better than the historical group. When the progression-free survival benefit was realized, it was no longer ethical to not offer sunitinib to the patients on the control arm, so in 2006 patients whose disease progressed on interferon could switch to sunitinib.
![]() DR LOVE: How did the data compare when you eliminated the patients who
switched from interferon to sunitinib?
DR LOVE: How did the data compare when you eliminated the patients who
switched from interferon to sunitinib?
![]() DR FIGLIN: When we analyzed the data for patients who only received
sunitinib compared to those who only received interferon, the survival was 28
versus 14 months, respectively.
DR FIGLIN: When we analyzed the data for patients who only received
sunitinib compared to those who only received interferon, the survival was 28
versus 14 months, respectively.
It’s difficult to measure survival when paradigm shifts are taking place. One third of the patients treated with interferon had received sunitinib at some time, either on or off study, and 59 percent received some poststudy treatment, such as sunitinib, sorafenib, mTOR inhibitors, cytokines or chemotherapy.
Nonetheless, my conclusions are straightforward, which are that these data are the first clear and unequivocal demonstration of a survival benefit for patients with mRCC compared to our historical groups.
For those of us who have been treating kidney cancer for decades, this is the first time we can see patients living for more than two years with metastatic disease.
Approximately 80 percent of the patients will have some reduction in their tumor, in the absence of progression, during the course of their treatment with sunitinib (Motzer 2007). Thus, when patients ask me what the likelihood is that they will benefit from sunitinib, I tell them that they have an eight-in-10 chance.
![]() DR LOVE: Do you feel that patients with mRCC are now living longer?
DR LOVE: Do you feel that patients with mRCC are now living longer?
![]() DR FIGLIN: Yes, our practices are growing because patients are living longer
and they’re coming back more frequently because they are staying on treatment
longer. With a median survival of two years, some patients are living significantly longer. I have a patient who participated in the original Phase II
trial and is still on sunitinib four and a half years later — her disease is under
control, and she’s leading a great life.
DR FIGLIN: Yes, our practices are growing because patients are living longer
and they’re coming back more frequently because they are staying on treatment
longer. With a median survival of two years, some patients are living significantly longer. I have a patient who participated in the original Phase II
trial and is still on sunitinib four and a half years later — her disease is under
control, and she’s leading a great life.
![]() DR LOVE: Is she in complete clinical remission?
DR LOVE: Is she in complete clinical remission?
![]() DR FIGLIN: No, she must be maintained on the drug. If we cut back, the
tumor grows. That’s important for practicing oncologists to understand. These
responses and this benefit are what we would characterize as maintained
remissions, not unmaintained remissions. The patients must continue on these
drugs, otherwise the tumor will start to grow again.
DR FIGLIN: No, she must be maintained on the drug. If we cut back, the
tumor grows. That’s important for practicing oncologists to understand. These
responses and this benefit are what we would characterize as maintained
remissions, not unmaintained remissions. The patients must continue on these
drugs, otherwise the tumor will start to grow again.
![]() DR LOVE: With this particular patient, what toxicities has she had to deal
with during the past four and a half years?
DR LOVE: With this particular patient, what toxicities has she had to deal
with during the past four and a half years?
![]() DR FIGLIN: Her major toxicity has been fatigue, followed by hand-foot
syndrome. She’s receiving 37.5 milligrams rather than the full 50 milligrams
because of these side effects, and at that dose she’s able to maintain an active
and full life.
DR FIGLIN: Her major toxicity has been fatigue, followed by hand-foot
syndrome. She’s receiving 37.5 milligrams rather than the full 50 milligrams
because of these side effects, and at that dose she’s able to maintain an active
and full life.
Track 9
![]() DR LOVE: You participated in the study that evaluated sorafenib in older
patients with advanced renal cell carcinoma. What did that trial show, and
what is your approach in practice?
DR LOVE: You participated in the study that evaluated sorafenib in older
patients with advanced renal cell carcinoma. What did that trial show, and
what is your approach in practice?
![]() DR FIGLIN: We found no apparent difficulty administering sorafenib to
patients older than age 65 compared to the population of patients who are
younger than age 65 (Bukowski 2008; [3.1]).
DR FIGLIN: We found no apparent difficulty administering sorafenib to
patients older than age 65 compared to the population of patients who are
younger than age 65 (Bukowski 2008; [3.1]).
In my practice, when I discuss sorafenib versus sunitinib with an elderly, asymptomatic patient, I explain that with one therapy, we may have to sacrifice benefit a bit, but a better quality-of-life component exists. Some patients want the more effective therapy, regardless of the toxicity, whereas others want a good quality of life and know that the other therapy will be available later if they need it.
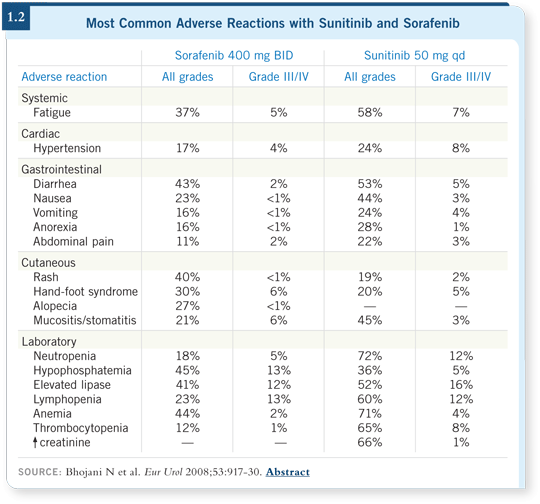
![]() DR LOVE: Which is better tolerated by older patients, sorafenib or sunitinib (1.2)?
DR LOVE: Which is better tolerated by older patients, sorafenib or sunitinib (1.2)?
![]() DR FIGLIN: In my experience, sorafenib is better tolerated by older patients.
We see less fatigue, hand-foot syndrome and hypertension in our patients who
are treated with sorafenib.
DR FIGLIN: In my experience, sorafenib is better tolerated by older patients.
We see less fatigue, hand-foot syndrome and hypertension in our patients who
are treated with sorafenib.
Track 12
![]() DR LOVE: What do you consider the optimal treatment for patients with
clear cell mRCC in the front-line setting?
DR LOVE: What do you consider the optimal treatment for patients with
clear cell mRCC in the front-line setting?
![]() DR FIGLIN: Level I evidence tells us that the treatment of choice is sunitinib.
Level I evidence also exists for the combination of bevacizumab and interferon
in the untreated patient population.
DR FIGLIN: Level I evidence tells us that the treatment of choice is sunitinib.
Level I evidence also exists for the combination of bevacizumab and interferon
in the untreated patient population.
![]() DR LOVE: What about bevacizumab alone for these patients?
DR LOVE: What about bevacizumab alone for these patients?
![]() DR FIGLIN: Bevacizumab monotherapy has never been tested in Phase III
trials. However, many people believe that interferon doesn’t contribute much
to the combination. Consider another observation published by Bernard
Escudier with regard to the combination (Melichar 2008). He presented data
comparing progression-free survival among patients who had received full
doses of interferon versus reduced doses.
DR FIGLIN: Bevacizumab monotherapy has never been tested in Phase III
trials. However, many people believe that interferon doesn’t contribute much
to the combination. Consider another observation published by Bernard
Escudier with regard to the combination (Melichar 2008). He presented data
comparing progression-free survival among patients who had received full
doses of interferon versus reduced doses.
Much to our surprise, patients who received the lower doses had a longer progression-free survival, so we don’t know whether the dose of interferon used when combined with bevacizumab needs to be that high. We may need to revisit the question of whether lower-dose interferon may be equally effective or possibly even more effective.
![]() DR LOVE: What did the clinical trial of bevacizumab with or without
erlotinib show?
DR LOVE: What did the clinical trial of bevacizumab with or without
erlotinib show?
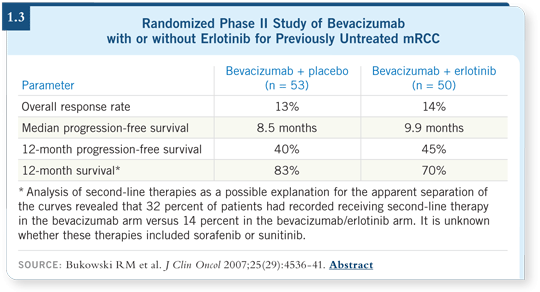
![]() DR FIGLIN: This was a randomized, Phase II study in mRCC, and it indicated
that the addition of erlotinib did not provide additional clinical benefit. It also
showed that bevacizumab monotherapy had a response rate that is lower and a
progression-free survival rate that appears inferior to what we expect with the
combination of bevacizumab and interferon, at least when compared study to
study (Bukowski 2007; [1.3]).
DR FIGLIN: This was a randomized, Phase II study in mRCC, and it indicated
that the addition of erlotinib did not provide additional clinical benefit. It also
showed that bevacizumab monotherapy had a response rate that is lower and a
progression-free survival rate that appears inferior to what we expect with the
combination of bevacizumab and interferon, at least when compared study to
study (Bukowski 2007; [1.3]).
Track 14
![]() DR LOVE: What is your current management algorithm for patients with
mRCC?
DR LOVE: What is your current management algorithm for patients with
mRCC?
![]() DR FIGLIN: My paradigm is simple. If a patient has a carcinoma with predominantly
clear cell features, whether the prognosis is good, intermediate or poor,
my treatment of choice is sunitinib.
DR FIGLIN: My paradigm is simple. If a patient has a carcinoma with predominantly
clear cell features, whether the prognosis is good, intermediate or poor,
my treatment of choice is sunitinib.
Temsirolimus is another option for the untreated patient. This agent is now commercially available for patients with poor prognostic features, such as a lower hemoglobin level, poorer performance status, multiple sites of metastatic disease or high-corrected calcium. In my practice, if a patient has other features that worsen the prognosis, I use temsirolimus.
![]() DR LOVE: What do you use for patients with nonclear cell histologies?
DR LOVE: What do you use for patients with nonclear cell histologies?
![]() DR FIGLIN: The temsirolimus trial with untreated patients included nonclear
cell histologies (Hudes 2007). In that patient population, even though
sunitinib is available, one has to consider temsirolimus.
DR FIGLIN: The temsirolimus trial with untreated patients included nonclear
cell histologies (Hudes 2007). In that patient population, even though
sunitinib is available, one has to consider temsirolimus.
Track 18
![]() DR LOVE: Are you participating in the Intergroup adjuvant trial (ECOGE2805)
evaluating sorafenib versus sunitinib versus placebo?
DR LOVE: Are you participating in the Intergroup adjuvant trial (ECOGE2805)
evaluating sorafenib versus sunitinib versus placebo?
![]() DR FIGLIN: At the City of Hope, we have entered many patients on this
spectacular trial. Already more than 800 patients are enrolled, with a target
accrual of 1,300. This is an interesting trial because treating a patient with
these targeted therapies in the adjuvant setting is different than in the
metastatic setting. Patients receiving adjuvant therapy have different expectations,
and their tolerance for toxicity is different.
DR FIGLIN: At the City of Hope, we have entered many patients on this
spectacular trial. Already more than 800 patients are enrolled, with a target
accrual of 1,300. This is an interesting trial because treating a patient with
these targeted therapies in the adjuvant setting is different than in the
metastatic setting. Patients receiving adjuvant therapy have different expectations,
and their tolerance for toxicity is different.
![]() DR LOVE: Have you used either of these agents as adjuvant therapy outside of
a clinical trial?
DR LOVE: Have you used either of these agents as adjuvant therapy outside of
a clinical trial?
![]() DR FIGLIN: I have not done so, and I will not do so. First we need to learn
whether these targeted agents work in the adjuvant setting. Theoretically, the
adjuvant setting includes patients with micrometastatic disease, and we have
not yet determined the role of anti-angiogenesis in micrometastatic disease. It
could be that angiogenesis isn’t as robust and, as such, can’t be inhibited as well
in that micrometastatic setting.
DR FIGLIN: I have not done so, and I will not do so. First we need to learn
whether these targeted agents work in the adjuvant setting. Theoretically, the
adjuvant setting includes patients with micrometastatic disease, and we have
not yet determined the role of anti-angiogenesis in micrometastatic disease. It
could be that angiogenesis isn’t as robust and, as such, can’t be inhibited as well
in that micrometastatic setting.
Editor
Neil Love, MD
Interviews
Robert A Figlin, MD
- Select publications
Gary R Hudes, MD
- Select publications
Ronald M Bukowski, MD
- Select publications

| Terms of Use and General Disclaimer | Privacy Policy Copyright © 2008 Research To Practice. All Rights Reserved. |