
 |
|||||

| Tracks 1-17 | ||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 5
![]() DR LOVE: Can you discuss your presentation at ASCO 2008 evaluating
sorafenib in older patients?
DR LOVE: Can you discuss your presentation at ASCO 2008 evaluating
sorafenib in older patients?
![]() DR BUKOWSKI: It was an analysis of the Advanced Renal Cell Carcinoma
Sorafenib (ARCCS) Expanded Access program. The question has been whether
older patients experience the same benefits and toxicities as younger patients.
The real issue is the definition of old, and no one agrees. The cutoff of 65 years
old was chosen (Bukowski 2008). In this study with more than 2,500 patients
enrolled by community doctors, we evaluated whether older patients and
younger patients had similar progression-free survival (PFS) rates and toxicities
associated with sorafenib.
DR BUKOWSKI: It was an analysis of the Advanced Renal Cell Carcinoma
Sorafenib (ARCCS) Expanded Access program. The question has been whether
older patients experience the same benefits and toxicities as younger patients.
The real issue is the definition of old, and no one agrees. The cutoff of 65 years
old was chosen (Bukowski 2008). In this study with more than 2,500 patients
enrolled by community doctors, we evaluated whether older patients and
younger patients had similar progression-free survival (PFS) rates and toxicities
associated with sorafenib.
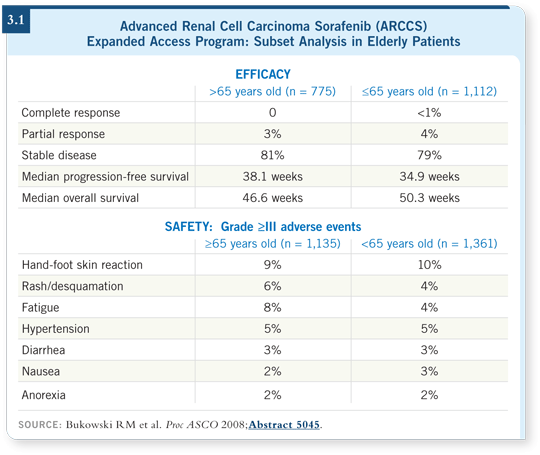
It turns out that they did. An increase in toxicities did not appear in the individuals older than age 65 (Bukowski 2008; [3.1]).
Track 6
![]() DR LOVE: Would you review the AVOREN trial and the update
presented at ASCO 2008?
DR LOVE: Would you review the AVOREN trial and the update
presented at ASCO 2008?
![]() DR BUKOWSKI: The AVOREN trial was a straightforward study in which
bevacizumab/interferon was compared to interferon alone as first-line therapy
for mRCC. Last year, the presentation focused on efficacy and demonstrated
that PFS was basically doubled when combining bevacizumab with interferon.
The response rate was likewise doubled from 15 to 30 percent (Escudier 2007).
DR BUKOWSKI: The AVOREN trial was a straightforward study in which
bevacizumab/interferon was compared to interferon alone as first-line therapy
for mRCC. Last year, the presentation focused on efficacy and demonstrated
that PFS was basically doubled when combining bevacizumab with interferon.
The response rate was likewise doubled from 15 to 30 percent (Escudier 2007).
In the presentation this year, Dr Escudier evaluated the patients who had interferon dose reductions. As one would expect, patients tolerated lower doses of interferon better than higher doses. Outcomes were also studied. Although it was a subset analysis, which needs to be conducted in that context, it showed no difference between patients who had interferon dose reductions and those who did not (Escudier 2008; Melichar 2008; [3.2]).
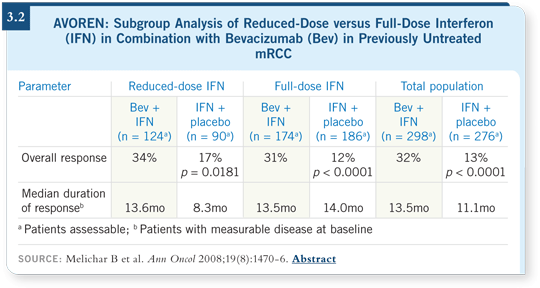
Is a lower dose of interferon sufficient and clearly easier to tolerate than the one used in the study? These data support that. They even address the issue of whether interferon is completely necessary.
As you reduce the dose, perhaps you can almost eliminate the drug. We cannot, however, do so at this point. So lower doses of interferon are acceptable. I believe that most physicians who have used interferon recognize that you can administer two or three million units three times per week easily compared to nine or 18 million units, with which the toxicity is much higher.
Track 7
![]() DR LOVE: You led a study evaluating bevacizumab alone versus bevacizumab
with erlotinib. How much do you think interferon is contributing
to the activity of bevacizumab?
DR LOVE: You led a study evaluating bevacizumab alone versus bevacizumab
with erlotinib. How much do you think interferon is contributing
to the activity of bevacizumab?
![]() DR BUKOWSKI: I wish we had an answer because it would make our lives
easier as we begin to use bevacizumab for renal cancer in the United States.
All we have are the data I presented and published, which included approximately
100 patients — half received bevacizumab alone, and the other half
received bevacizumab and erlotinib (Bukowski 2007; [1.3]).
DR BUKOWSKI: I wish we had an answer because it would make our lives
easier as we begin to use bevacizumab for renal cancer in the United States.
All we have are the data I presented and published, which included approximately
100 patients — half received bevacizumab alone, and the other half
received bevacizumab and erlotinib (Bukowski 2007; [1.3]).
Erlotinib didn’t have any effect except the usual toxicity. The median PFS for the group as a whole was approximately nine months. We saw little difference in the median PFS between the two arms — one was a little less than nine months and the other a little more than nine months. The response rates in both arms were in the range of 13 to 14 percent (Bukowski 2007; [1.3]). This would be our de facto bevacizumab monotherapy experience that is available in a randomized, blinded setting.
When we view the data from AVOREN (Escudier 2007) and CALGB-90206 (Rini 2008; [3.3]) — two large groups of patients treated with the combination of bevacizumab and interferon — both show an effect on progression.
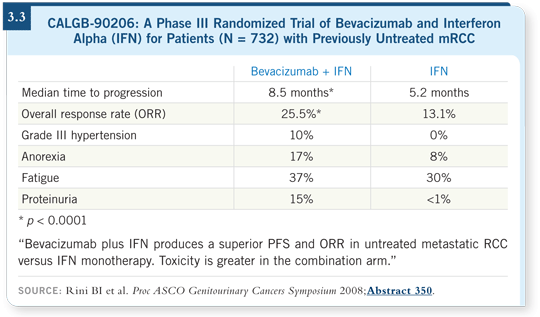
In the AVOREN study, the median PFS was approximately 10.2 months. In CALGB-90206, the median time to progression was 8.5 months.
You come away thinking that monotherapy with bevacizumab, if indeed one can extrapolate from the erlotinib/bevacizumab trial, is almost equivalent with perhaps a 1.5-month lower median PFS than with the combination of interferon and bevacizumab. You have the sense that the major contribution is from bevacizumab. My impression is that if we had a well-designed study in which we used bevacizumab monotherapy, we would see a median PFS of nine to 10 months.
Track 16
![]() DR LOVE: Which new research strategies are currently receiving high
priority?
DR LOVE: Which new research strategies are currently receiving high
priority?
![]() DR BUKOWSKI: The data that keep coming out are with combinations, which
is the next frontier. Can we combine these drugs in a fashion that enhances
efficacy? We don’t know. I caution people against prematurely using combinations
at this point because we have seen toxicity that is worrisome and
problematic.
DR BUKOWSKI: The data that keep coming out are with combinations, which
is the next frontier. Can we combine these drugs in a fashion that enhances
efficacy? We don’t know. I caution people against prematurely using combinations
at this point because we have seen toxicity that is worrisome and
problematic.
One paper, presented for the third time, was the Phase I trial of sorafenib and bevacizumab conducted by Jeff Sosman. The response rate remains robust at about 40 percent, but the toxicity is clearly problematic. One needs to reduce the doses of both bevacizumab and sorafenib to make the combination tolerable (Sosman 2008).
The other intriguing combination is sunitinib/bevacizumab, which has been studied at two centers — Memorial Sloan-Kettering (Feldman 2008) and the Cleveland Clinic (Cooney 2008). The outcomes at Memorial were such that you could administer full doses of both drugs, but within a cycle or two, toxicity developed that was a problem.
We saw three or four cases of hemolytic uremic syndrome, which is difficult to manage and could be life threatening. The combination at full doses was abandoned in terms of further studies.
At the Cleveland Clinic, we’ve not seen a single case of hemolytic uremic syndrome, but many of our patients, as they continue therapy, have had dose reductions because of other toxicities. You come away thinking that the tyrosine kinase inhibitors sorafenib and sunitinib are sometimes difficult to combine with other agents.
However, bevacizumab appears to be a drug that you can combine with other agents, especially with the mTOR inhibitors. A lot of interest exists for the combinations of bevacizumab with temsirolimus or everolimus.
Right now, it’s fair to say that the data on combinations are preliminary. We don’t have evidence to say that they are tolerated long term or that efficacy will be improved.
Editor
Neil Love, MD
Interviews
Robert A Figlin, MD
- Select publications
Gary R Hudes, MD
- Select publications
Ronald M Bukowski, MD
- Select publications

| Terms of Use and General Disclaimer | Privacy Policy Copyright © 2008 Research To Practice. All Rights Reserved. |