
 |
|||||

| Tracks 1-16 | ||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1-2
![]() DR MOTZER: Renal cell cancer afflicts approximately 50,000 people each year
in the US and usually occurs in individuals in their sixties. It’s the eighth most
common cancer in men, with a man-to-woman ratio of two to one.
DR MOTZER: Renal cell cancer afflicts approximately 50,000 people each year
in the US and usually occurs in individuals in their sixties. It’s the eighth most
common cancer in men, with a man-to-woman ratio of two to one.
The standard curative treatment for RCC for many years has been surgical resection of the primary kidney tumor for localized disease. However, RCC is considered a “silent cancer,” and often no symptoms occur that lead to early diagnosis. Many patients present with metastatic disease at initial diagnosis or relapse with metastases after a nephrectomy. For these individuals, this was for many years considered an especially difficult cancer to treat.
Different chemotherapies were tried, and none provided meaningful clinical benefit. It was considered the model for chemotherapy-resistant cancer.
Until recently, the mainstay of systemic treatment for metastases was immunotherapy with the cytokines — interferon and interleukin-2. Both were recognized in the 1980s as having some level of activity, and until recently they remained the only agents that showed activity in this disease.
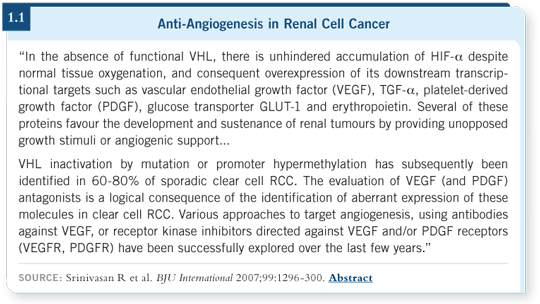
In the 1990s, through careful work by laboratory-based physicians, a gene was recognized — the von Hippel-Lindau gene — that’s frequently mutated and inactive in RCC (1.1). The downstream effects of the gene are to prevent tumor growth and blood vessel formation.
As anti-angiogenic targeted therapies were developed, RCC was recognized as an important cancer in which to study these agents. These anti-angiogenic treatments are paying off and have now been implemented as the standard treatment for RCC.
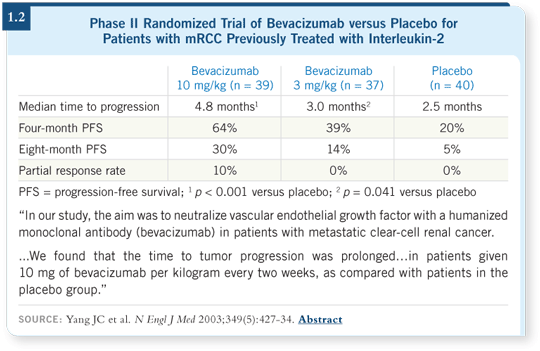
The newer targeted therapies were initially studied in patients who had been treated with interferon or interleukin-2 and had progressive disease. Bevacizumab — a neutralizing antibody to vascular endothelial growth factor (VEGF) — was the first agent to be studied in a small, randomized trial with patients whose disease progressed on high-dose interleukin-2 (Yang 2003; [1.2]).
Dr Yang and colleagues from the NCI reported a statistically significant improvement in progression-free survival for the patients treated with bevacizumab at 10 mg/kg every two weeks compared to placebo. This study served as a proof of principle that we were on the right track with targeted antiangiogenic therapies in mRCC.
Tracks 3-5
![]() DR LOVE: Can you discuss the development of TKIs for the treatment
of RCC?
DR LOVE: Can you discuss the development of TKIs for the treatment
of RCC?
![]() DR MOTZER: The next agent studied was sorafenib, a multitargeted TKI,
which is believed to affect RCC through inhibition of VEGF receptor and
platelet-derived growth factor (PDGF) receptor.
DR MOTZER: The next agent studied was sorafenib, a multitargeted TKI,
which is believed to affect RCC through inhibition of VEGF receptor and
platelet-derived growth factor (PDGF) receptor.
Sorafenib was initially evaluated in a randomized, Phase II discontinuation study (Ratain 2006), which is a trial design utilized to evaluate drugs that are expected to effect disease stabilization or to prolong progression-free survival rather than produce classic tumor responses. Approximately two thirds of the patients experienced some degree of tumor shrinkage. In fact, the study demonstrated a prolongation in progression-free survival for sorafenib compared to placebo (Ratain 2006).
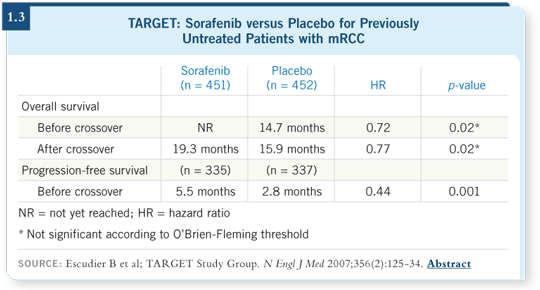
That study led to a large, randomized, Phase III trial (TARGET) in which patients with clear cell histology whose disease progressed on prior therapy (interferon or interleukin) were randomly assigned to sorafenib versus placebo (Escudier 2007).
This was the largest trial conducted worldwide in RCC and included more than 900 patients. The response rate was predictably about 10 percent for sorafenib, but progression-free survival was approximately doubled with sorafenib compared to placebo (1.3). The trial was positive, and the patients on the placebo arm were allowed a crossover to sorafenib.
![]() DR LOVE: How do those data compare to what you observed with sunitinib?
DR LOVE: How do those data compare to what you observed with sunitinib?
![]() DR MOTZER: The first sunitinib study was a single-arm trial conducted at five
centers with 63 patients whose disease had progressed on first-line cytokine
therapy, with sunitinib administered at the standard dose of 50 milligrams
daily oral therapy for four weeks followed by two weeks off.
DR MOTZER: The first sunitinib study was a single-arm trial conducted at five
centers with 63 patients whose disease had progressed on first-line cytokine
therapy, with sunitinib administered at the standard dose of 50 milligrams
daily oral therapy for four weeks followed by two weeks off.
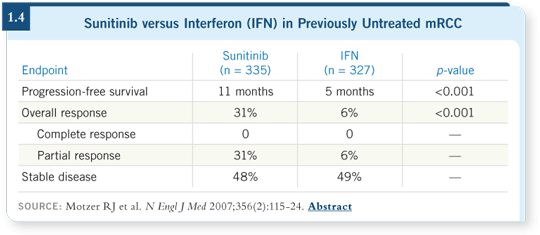
Approximately 40 percent of the patients achieved a partial response, and the median progression-free survival was 8.7 months, which compared favorably to the two to three months that would be expected with inactive agents as part of the historical control (Motzer 2006a). A larger, single-arm pivotal trial was conducted with response as the primary endpoint in second-line treatment (Motzer 2006b), and a large Phase III trial compared sunitinib to interferon alpha as first-line therapy (Motzer 2007; [1.4]).
![]() DR LOVE: What about temsirolimus and its role in the treatment of RCC?
DR LOVE: What about temsirolimus and its role in the treatment of RCC?
![]() DR MOTZER: Temsirolimus is a unique anticancer agent. It’s an mTOR
(mammalian target of rapamycin) inhibitor and is the first drug in this class
of agents approved for the treatment of advanced RCC. It was originally
studied in RCC as part of a randomized Phase II trial of three different dose levels that were administered to heavily pretreated patients (Atkins 2004). The
response rate was approximately 10 percent, but many patients experienced
stabilization of disease, with a median progression-free survival of six months
and an overall survival of approximately 12 months (Atkins 2004).
DR MOTZER: Temsirolimus is a unique anticancer agent. It’s an mTOR
(mammalian target of rapamycin) inhibitor and is the first drug in this class
of agents approved for the treatment of advanced RCC. It was originally
studied in RCC as part of a randomized Phase II trial of three different dose levels that were administered to heavily pretreated patients (Atkins 2004). The
response rate was approximately 10 percent, but many patients experienced
stabilization of disease, with a median progression-free survival of six months
and an overall survival of approximately 12 months (Atkins 2004).
These results were better than for the historical control group, and subset analyses indicated that patients with poor-risk disease — according to the Memorial Sloan-Kettering classification — received the most benefit.
This led to a large, pivotal, Phase III trial of temsirolimus versus interferon alpha versus the combination as first-line treatment for patients with poor-risk features (Hudes 2007). The study was not confined to clear cell carcinoma and included patients with other renal cancer histologies. The second interim analysis demonstrated an improvement in survival for temsirolimus over interferon alpha or the combination of temsirolimus and interferon (Hudes 2007; [4.1]).
Track 15
![]() DR LOVE: Over the past three to four years, how much improvement
have you observed in survival and quality of life in the metastatic setting?
DR LOVE: Over the past three to four years, how much improvement
have you observed in survival and quality of life in the metastatic setting?
![]() DR MOTZER: The discovery of targeted therapies represents dramatic progress
in this disease. In renal cancer, we anticipate a situation similar to what we see
in several of the other types of cancer in which a patient may benefit from one
of these targeted therapies for a time, then experience progression. We’ll be able
to offer the patient a second, a third and potentially a fourth therapy. I believe
we will see a dramatic change in overall survival for patients with metastatic
renal cancer using multiple targeted therapies.
DR MOTZER: The discovery of targeted therapies represents dramatic progress
in this disease. In renal cancer, we anticipate a situation similar to what we see
in several of the other types of cancer in which a patient may benefit from one
of these targeted therapies for a time, then experience progression. We’ll be able
to offer the patient a second, a third and potentially a fourth therapy. I believe
we will see a dramatic change in overall survival for patients with metastatic
renal cancer using multiple targeted therapies.
| Terms of Use and General Disclaimer | Privacy Policy Copyright © 2008 Research To Practice. All Rights Reserved. |