
 |
|||||

| Tracks 1-18 | ||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Whatís your take on the safety and toxicity issues with the
major options for first-line treatment of metastatic RCC?
DR LOVE: Whatís your take on the safety and toxicity issues with the
major options for first-line treatment of metastatic RCC?
![]() DR AMATO: Thatís a good question. Letís break it down. The predominant
side effect with sunitinib is fatigue, thus the built-in break ó four weeks on,
two weeks off. Additional side effects occur, from skin reactions to gastrointestinal
reactions, and occasionally youíll obtain a hematologic profile with a
decrease in counts. Those patients need the two-week rest period.
DR AMATO: Thatís a good question. Letís break it down. The predominant
side effect with sunitinib is fatigue, thus the built-in break ó four weeks on,
two weeks off. Additional side effects occur, from skin reactions to gastrointestinal
reactions, and occasionally youíll obtain a hematologic profile with a
decrease in counts. Those patients need the two-week rest period.
The clear issue with sorafenib is skin reactions associated with hand-foot syndrome. With both of these drugs, if you begin manipulating by lowering the dose to adjust for side-effect toxicity, you tend to lose efficacy. So you need to maintain at the approved doses.
Bevacizumab as a single agent is more manageable. Patients may exhibit some hypertension and proteinuria, but those are more manageable. With the addition of interferon to bevacizumab, the side-effect profile remains similar. If one evaluates this strictly from a tolerability standpoint, it would be fair to make the statement that interferon/bevacizumab is better tolerated than the oral TKIs.
![]() DR LOVE: What about efficacy?
DR LOVE: What about efficacy?
![]() DR AMATO: It is unfair to say one is better than the other without conducting
head-to-head trials. But if you compare across the board, they are similar. Phase
II and Phase III data with sunitinib indicate anywhere from 30 to 40 percent
activity, with an additional disease-stabilization portion (Motzer 2006a, 2007).
DR AMATO: It is unfair to say one is better than the other without conducting
head-to-head trials. But if you compare across the board, they are similar. Phase
II and Phase III data with sunitinib indicate anywhere from 30 to 40 percent
activity, with an additional disease-stabilization portion (Motzer 2006a, 2007).
In trials evaluating sorafenib, activity is somewhere between five and 10 percent, but again with that stability portion (Escudier 2007c). Studies of interferon/bevacizumab reported approximately a 30 percent tumor response rate, again with a stability fraction (Escudier 2007b). With regard to progression- free survival, theyíre all between five and 10 months.
Track 4
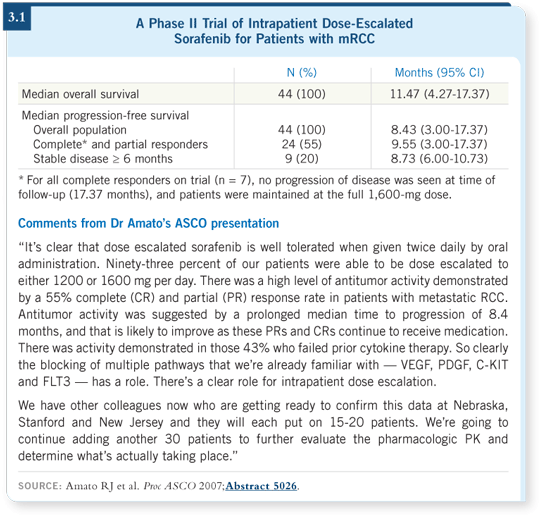
![]() DR LOVE: Can you talk about your presentation at ASCO evaluating dose
escalation of sorafenib (Amato 2007; [3.1])?
DR LOVE: Can you talk about your presentation at ASCO evaluating dose
escalation of sorafenib (Amato 2007; [3.1])?
![]() DR AMATO: The trial was for patients with clear cell component mRCC, who
were started on sorafenib at 400 milligrams BID then had the dose escalated
according to a prescribed schedule.
DR AMATO: The trial was for patients with clear cell component mRCC, who
were started on sorafenib at 400 milligrams BID then had the dose escalated
according to a prescribed schedule.
The study was based on Phase I data suggesting that at higher doses, sorafenib exhibited more activity, leading to the hypothesis that you might have more kinase inhibition at higher doses.
We reported a 16 percent complete response rate among 44 patients. Seven of 44 patients had a complete response, and another 17 had a partial response ó a 55 percent objective response. The progression-free survival median was 8.4 months, which suggests that we now have a complete response rate population and partial response rate population similar to what we know of already, simply by increasing the dose.
![]() DR LOVE: Do you believe thatís a reasonable strategy to implement currently
in a clinical setting?
DR LOVE: Do you believe thatís a reasonable strategy to implement currently
in a clinical setting?
![]() DR AMATO: Not at the present time. We are confirming the data. All the
complete responders are approaching 15 to 24 months now, and we have added
another 30 patients. Tune in at the next ASCO, and we will have the answers.
DR AMATO: Not at the present time. We are confirming the data. All the
complete responders are approaching 15 to 24 months now, and we have added
another 30 patients. Tune in at the next ASCO, and we will have the answers.
| Terms of Use and General Disclaimer | Privacy Policy Copyright © 2008 Research To Practice. All Rights Reserved. |