
 |
|||||

| Tracks 1-18 | ||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 2
![]() DR LOVE: Can you discuss the available clinical research data for bevacizumab
in RCC?
DR LOVE: Can you discuss the available clinical research data for bevacizumab
in RCC?
![]() DR GEORGE: A trial by Jim Yang at the NCI was one of the first proof-of-concept
studies. In patients who had failed interleukin-2, bevacizumab at
10 mg/kg every two weeks almost doubled the duration of progression-free
survival compared to placebo. They were able to show that this real, dramatic change in the natural history of the disease was accompanied by relatively
few patients achieving a partial response (Yang 2003; [1.2]).
DR GEORGE: A trial by Jim Yang at the NCI was one of the first proof-of-concept
studies. In patients who had failed interleukin-2, bevacizumab at
10 mg/kg every two weeks almost doubled the duration of progression-free
survival compared to placebo. They were able to show that this real, dramatic change in the natural history of the disease was accompanied by relatively
few patients achieving a partial response (Yang 2003; [1.2]).
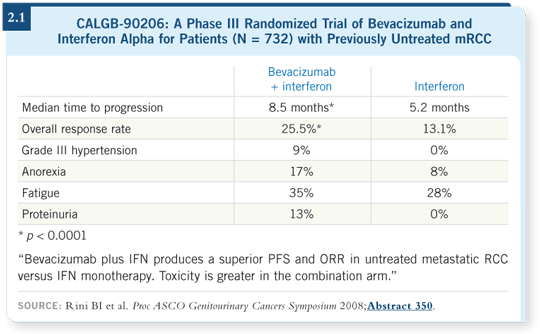
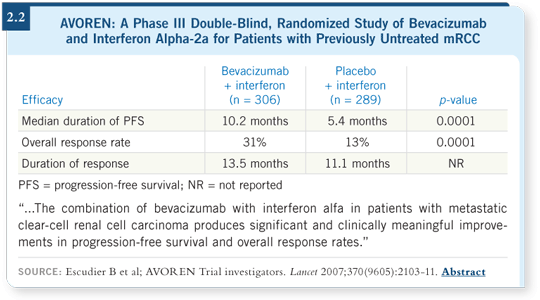
In the US, the CALGB conducted a large Phase III study (CALGB-90206) in which we compared bevacizumab with interferon to interferon alone. Those results showed a dramatic improvement in the duration of progression-free survival with the addition of bevacizumab (Rini 2008; [2.1]). In the CALGB study, the median time to progression for interferon alone was 5.2 months, and it was 8.5 months for interferon with bevacizumab (Rini 2008; [2.1]), which is similar to what we saw in the Roche study (AVOREN; [Escudier 2007; 2.2]).
In Europe, a similarly designed study (AVOREN) that included a placebo control ó interferon/placebo versus interferon/bevacizumab ó demonstrated similar results. An approximately 80 percent improvement was evident in median duration of progression-free survival, from 5.4 months for interferon/ placebo to 10.2 months for interferon/bevacizumab (Escudier 2007; [2.2]). Now we have two large, multicenter, Phase III studies showing a dramatic improvement in progression-free survival with bevacizumab.
Tracks 3-4
![]() DR LOVE: What about bevacizumab monotherapy for a patient with
asymptomatic or minimally symptomatic metastatic disease?
DR LOVE: What about bevacizumab monotherapy for a patient with
asymptomatic or minimally symptomatic metastatic disease?
![]() DR GEORGE: It probably has the least toxic profile. We donít see hand-foot
syndrome with bevacizumab. We also donít see diarrhea or gastrointestinal
toxicities. Fatigue is probably less significant than what we see with the other
targeted agents. Bevacizumab is probably the least toxic as a single agent
in terms of being able to have an anti-angiogenic biologic effect, delay the
progression of disease and change the natural history.
DR GEORGE: It probably has the least toxic profile. We donít see hand-foot
syndrome with bevacizumab. We also donít see diarrhea or gastrointestinal
toxicities. Fatigue is probably less significant than what we see with the other
targeted agents. Bevacizumab is probably the least toxic as a single agent
in terms of being able to have an anti-angiogenic biologic effect, delay the
progression of disease and change the natural history.
The key questions are, how much more effective are these other regimens, and does interferon sufficiently improve the progression-free survival when combined with bevacizumab to justify the months of interferon toxicity? These questions will be difficult to answer in a clinical trial. Thatís where the art of medicine comes in for a practicing clinician ó determining to what extent your patient can tolerate the side-effect profile and gain a benefit.
Some patients tolerate the combination well, but I believe they are the minority. Physicians may decide to try a combination of interferon/bevacizumab and discontinue or reduce the dose of interferon if and when toxicities occur. They may also decide to start with bevacizumab alone, and if patients are faring well, they may try administering interferon. These concepts arenít proven in a clinical trial setting but can be extrapolated from the existing data.
![]() DR LOVE: Do you have a sense of how much toxicity the cytokines add to
bevacizumab in this situation?
DR LOVE: Do you have a sense of how much toxicity the cytokines add to
bevacizumab in this situation?
![]() DR GEORGE: I believe it would be substantial. Approximately 80 percent of
the toxicities ó fever, chills, weight loss, cytopenias, et cetera ó are driven
by the cytokines. With bevacizumab alone, very few of those side effects are
evident. The side effects primarily seen with bevacizumab include fatigue,
hypertension and proteinuria, which patients can tolerate well in a chronic
setting without a lot of symptoms. I believe many physicians in the community
will attempt to use bevacizumab monotherapy.
DR GEORGE: I believe it would be substantial. Approximately 80 percent of
the toxicities ó fever, chills, weight loss, cytopenias, et cetera ó are driven
by the cytokines. With bevacizumab alone, very few of those side effects are
evident. The side effects primarily seen with bevacizumab include fatigue,
hypertension and proteinuria, which patients can tolerate well in a chronic
setting without a lot of symptoms. I believe many physicians in the community
will attempt to use bevacizumab monotherapy.
![]() DR LOVE: How much is the cytokine contributing to the efficacy that is seen
with the combination?
DR LOVE: How much is the cytokine contributing to the efficacy that is seen
with the combination?
![]() DR GEORGE: We donít have randomized data to answer the question definitively.
I will say that in both of these Phase III studies ó CALGB (Rini 2008;
[2.1]) and AVOREN (Escudier 2007; [2.2]) ó the partial response rate was in
the 20 to 25 percent range for the combination, which is higher than weíve
seen in any Phase III study of interferon alone, and it is higher than what weíve seen with bevacizumab alone in those relatively small Phase II studies
(Bukowski 2007; Yang 2003). I believe some effect of the cytokines is interacting
with bevacizumab, but to what extent does that justify the toxicity?
DR GEORGE: We donít have randomized data to answer the question definitively.
I will say that in both of these Phase III studies ó CALGB (Rini 2008;
[2.1]) and AVOREN (Escudier 2007; [2.2]) ó the partial response rate was in
the 20 to 25 percent range for the combination, which is higher than weíve
seen in any Phase III study of interferon alone, and it is higher than what weíve seen with bevacizumab alone in those relatively small Phase II studies
(Bukowski 2007; Yang 2003). I believe some effect of the cytokines is interacting
with bevacizumab, but to what extent does that justify the toxicity?
Track 12
![]() DR LOVE: Setting cost and reimbursement issues aside and focusing on
risk-benefit along with clinical science, could you discuss your current
algorithm for first- and second-line therapy for patients with metastatic
disease?
DR LOVE: Setting cost and reimbursement issues aside and focusing on
risk-benefit along with clinical science, could you discuss your current
algorithm for first- and second-line therapy for patients with metastatic
disease?
![]() DR GEORGE: My answers today are different than they were one year ago,
and they are likely to be different a year from now. When I first see my
patients, I ďsize them upĒ as having good-, intermediate- or poor-risk disease.
DR GEORGE: My answers today are different than they were one year ago,
and they are likely to be different a year from now. When I first see my
patients, I ďsize them upĒ as having good-, intermediate- or poor-risk disease.
For a young, healthy patient with asymptomatic, low-volume disease who experienced a delay from the original diagnosis to the time of metastasis (ie, good-risk disease), I may discuss high-dose interleukin-2. Although we donít have a durable, long-term, disease-free control or cure rate, we know that a subset of patients will have a complete response. I believe itís fair for patients to at least hear about interleukin-2, but I donít push anybody toward it. Interleukin- 2 is an extremely toxic approach, and you need motivated patients in order to get them through the treatment.
Then weíll talk about some of our other agents. The agent we have the best data with in the first-line setting is sunitinib. In a Phase III study, the subpopulation with good-risk disease showed a dramatic progression-free survival benefit with sunitinib compared to interferon. The hazard ratio was 0.37 (Motzer 2007). So thatís a population in which Iíll either consider watchful waiting or sunitinib.
Bevacizumab at every other-week dosing would be an alternative. In a patient population that may be on a drug for a year or more, weíll talk about bevacizumab. So in that population, weíll start with those two agents. Sorafenib and temsirolimus are agents Iíd consider in the second- or third-line setting.
| Terms of Use and General Disclaimer | Privacy Policy Copyright © 2008 Research To Practice. All Rights Reserved. |