
 |
|||||

| Tracks 1-14 | ||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 4
![]() DR STADLER: Kidney cancer is distinctive in that these tumors express one of
the highest levels of VEGF.
DR STADLER: Kidney cancer is distinctive in that these tumors express one of
the highest levels of VEGF.
This is directly related to the pathophysiology of this tumor type, which is thought to involve inactivation of the von Hippel-Lindau (VHL) pathway leading to upregulation of the HIF transcription factor, which in turn leads to upregulation of VEGF.
These data, which were obtained from expression profiling, suggest a direct anti-angiogenic effect as the mechanism of action of bevacizumab in kidney cancer.
Tracks 5-6
![]() DR STADLER: For patients with good prognoses, the most mature front-line
data are with the VEGF receptor TKIs — specifically with sunitinib — so that
would be my choice for first-line therapy. That’s also driven by the fact that as
of now, bevacizumab has not received full regulatory approval for treatment. I
believe that once bevacizumab receives approval, whether one uses the TKIs,
bevacizumab or temsirolimus first line remains an open question.
DR STADLER: For patients with good prognoses, the most mature front-line
data are with the VEGF receptor TKIs — specifically with sunitinib — so that
would be my choice for first-line therapy. That’s also driven by the fact that as
of now, bevacizumab has not received full regulatory approval for treatment. I
believe that once bevacizumab receives approval, whether one uses the TKIs,
bevacizumab or temsirolimus first line remains an open question.
In the second line, we enroll patients on one of our trials. We’re considering the use of alternative VEGF receptor-targeting agents but also have a trial evaluating combinations of agents with bevacizumab. One of the combinations we’re studying is bevacizumab with gemcitabine and capecitabine, based on earlier work we conducted at our institution and through the CALGB.
![]() DR LOVE: How would you ideally use it in renal cell cancer?
DR LOVE: How would you ideally use it in renal cell cancer?
![]() DR STADLER: Using it in the front line makes sense, especially for patients
with good prognoses. My recommendation would be to use bevacizumab
with interferon because that’s what the Phase III data tell us (Escudier 2007).
DR STADLER: Using it in the front line makes sense, especially for patients
with good prognoses. My recommendation would be to use bevacizumab
with interferon because that’s what the Phase III data tell us (Escudier 2007).
![]() DR LOVE: How would you compare quality of life using sorafenib or sunitinib
versus bevacizumab as a single agent?
DR LOVE: How would you compare quality of life using sorafenib or sunitinib
versus bevacizumab as a single agent?
![]() DR STADLER: I believe the toxicities of sorafenib and sunitinib are broader
than the toxicities of bevacizumab, in that the TKIs also produce skin and
gastrointestinal (GI) toxicities. Certainly some patients have more difficulty
with some of these skin and bowel toxicities from the TKIs, but many of our
patients have tolerated these treatments for long periods.
DR STADLER: I believe the toxicities of sorafenib and sunitinib are broader
than the toxicities of bevacizumab, in that the TKIs also produce skin and
gastrointestinal (GI) toxicities. Certainly some patients have more difficulty
with some of these skin and bowel toxicities from the TKIs, but many of our
patients have tolerated these treatments for long periods.
Track 7
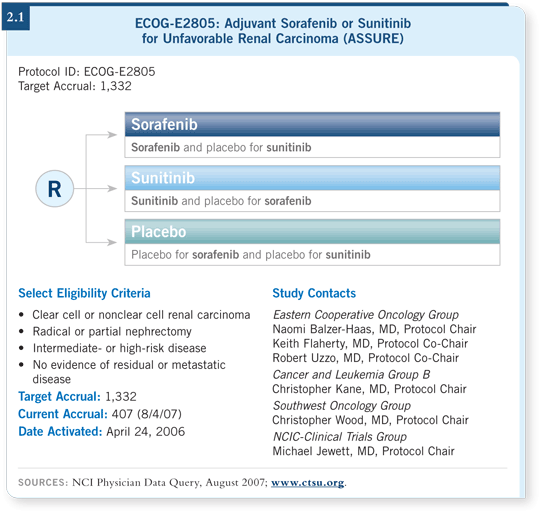
![]() DR STADLER: This is an ECOG-sponsored trial (ECOG-E2805) for patients
with high-risk cancer after surgery and no evidence of metastatic disease (2.1).
Patients are randomly assigned to one year of sorafenib versus sunitinib versus
placebo, with disease-free survival as the primary endpoint. The study also
includes an opportunity to register patients prior to surgery in order to collect
tumor specimens and evaluate potential molecular predictive markers.
DR STADLER: This is an ECOG-sponsored trial (ECOG-E2805) for patients
with high-risk cancer after surgery and no evidence of metastatic disease (2.1).
Patients are randomly assigned to one year of sorafenib versus sunitinib versus
placebo, with disease-free survival as the primary endpoint. The study also
includes an opportunity to register patients prior to surgery in order to collect
tumor specimens and evaluate potential molecular predictive markers.
![]() DR LOVE: In this study, is it going to be evident which patients are receiving
sorafenib or sunitinib and which patients are receiving placebo?
DR LOVE: In this study, is it going to be evident which patients are receiving
sorafenib or sunitinib and which patients are receiving placebo?
![]() DR STADLER: I believe there is a concern that this study is not going to be
truly blinded. Obviously if a patient has significant skin or diarrheal toxicities,
it’s unlikely that the patient is receiving placebo. However, we’ve learned in conducting placebo-controlled trials that our accuracy in predicting which
agent the patient was receiving is much less than we thought it would be.
DR STADLER: I believe there is a concern that this study is not going to be
truly blinded. Obviously if a patient has significant skin or diarrheal toxicities,
it’s unlikely that the patient is receiving placebo. However, we’ve learned in conducting placebo-controlled trials that our accuracy in predicting which
agent the patient was receiving is much less than we thought it would be.
![]() DR LOVE: Do you have any predictions as to the outcome of the trial?
DR LOVE: Do you have any predictions as to the outcome of the trial?
![]() DR STADLER: I’m hopeful that the data will show that one of the oral TKIs
will delay disease progression.
DR STADLER: I’m hopeful that the data will show that one of the oral TKIs
will delay disease progression.
Track 11
![]() DR STADLER: A lot of interest has been expressed with regard to combining
VEGF pathway targeted agents — either the TKIs or bevacizumab — with the
mTor (mammalian target of rapamycin) inhibitors, such as sirolimus, temsirolimus
and everolimus. There is great interest in combining these classes
of agents because they work on different but related pathways. ECOG is
launching a randomized Phase II trial with multiple combinations, including
bevacizumab, sorafenib and temsirolimus in various combinations (2.2).
DR STADLER: A lot of interest has been expressed with regard to combining
VEGF pathway targeted agents — either the TKIs or bevacizumab — with the
mTor (mammalian target of rapamycin) inhibitors, such as sirolimus, temsirolimus
and everolimus. There is great interest in combining these classes
of agents because they work on different but related pathways. ECOG is
launching a randomized Phase II trial with multiple combinations, including
bevacizumab, sorafenib and temsirolimus in various combinations (2.2).
I believe the biggest question is whether these combinations will prove to be any better than using the drugs in sequence, because we know that the combinations increase toxicity.

| Terms of Use/Disclaimer | Privacy Policy | Hardware/Software Requirements Copyright © 2007 Research To Practice. All Rights Reserved. |