
 |
|||||

| Tracks 1-17 | ||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 1, 10
![]() DR QUINN: The AVOREN trial was a randomized Phase III study that evaluated
patients with previously untreated metastatic clear cell carcinoma. Patients
were randomly assigned to receive interferon three times a week in combination with either placebo or bevacizumab. Overall survival was the primary
endpoint.
DR QUINN: The AVOREN trial was a randomized Phase III study that evaluated
patients with previously untreated metastatic clear cell carcinoma. Patients
were randomly assigned to receive interferon three times a week in combination with either placebo or bevacizumab. Overall survival was the primary
endpoint.
A doubling in progression-free survival occurred among patients who received bevacizumab with interferon compared to interferon with placebo. However, prior to the release of these data, interferon usage in the United States had dropped to virtually zero, so the AVOREN data raise the question of where interferon fits into the equation.
For the practitioner wanting to use bevacizumab, the question is whether to combine it with interferon in the first line or consider data from Phase II studies, such as the study presented by Dr Bukowski last year in which they evaluated bevacizumab alone versus bevacizumab with erlotinib and found no advantage to adding erlotinib (Bukowski 2006). However, in the bevacizumab- alone arm, the progression-free survival rate was approximately eight months. If one considers the data with the angiogenesis inhibitors, progression- free survival is between six and 11 months, depending on the setting.
In the first-line setting in this category, we have a choice of agents: sorafenib, sunitinib and bevacizumab — whether alone or in combination. A practitioner could pick any one of these, and one could argue that it doesn’t matter which angiogenesis inhibitor you start with.
![]() DR LOVE: Bottom line, currently what are your first-, second- and third-line
therapies for patients with good-risk renal cell carcinoma?
DR LOVE: Bottom line, currently what are your first-, second- and third-line
therapies for patients with good-risk renal cell carcinoma?
![]() DR QUINN: Currently, I start the patient on either sorafenib or sunitinib.
The question is which TKI to start with. Sunitinib has the best data in the
first-line setting (Motzer 2007), and data from a randomized Phase II study
evaluating sorafenib did not indicate that sorafenib had greater activity than
interferon, although sorafenib did allow for a better quality of life (Escudier
2007b).
DR QUINN: Currently, I start the patient on either sorafenib or sunitinib.
The question is which TKI to start with. Sunitinib has the best data in the
first-line setting (Motzer 2007), and data from a randomized Phase II study
evaluating sorafenib did not indicate that sorafenib had greater activity than
interferon, although sorafenib did allow for a better quality of life (Escudier
2007b).
I believe older patients may tolerate sorafenib better than sunitinib because of fatigue issues. However, if I have a patient that cannot tolerate hand-foot syndrome because he or she has a dexterous job — playing in the orchestra, for example — then I steer away from sorafenib and administer sunitinib. After that, I apply an algorithm based on the disease control and how well the patient is tolerating the medication.
Some of the patients develop a rash, hand-foot syndrome or fatigue. In that setting, if I have a patient with stable disease or only a suggestion of progression, my threshold is now low for switching to the other TKI.
My approach is to “mix and match” based on the side effects experienced by the individual patient. We have evidence from the clinic and published in abstracts, but not tested specifically in studies, that sequential TKI inhibition can produce responses in patients with disease that was resistant or stable (Dham 2007; Sablin 2007).
Considering the data on bevacizumab/interferon-![]() 2a that were presented at
ASCO 2007 (Escudier 2007a), if I started with one of the TKIs and the patient didn’t do well, I might recommend a three-weekly infusion of bevacizumab.
However, I don’t believe that is standard.
2a that were presented at
ASCO 2007 (Escudier 2007a), if I started with one of the TKIs and the patient didn’t do well, I might recommend a three-weekly infusion of bevacizumab.
However, I don’t believe that is standard.
Track 13
![]() DR QUINN: It is fascinating. The patients started on a standard dose of
sorafenib — 400 milligrams twice a day for one month. If they did not
develop particular toxicities, the dose was escalated to 600 milligrams twice a
day and then after another month to 800 milligrams twice a day.
DR QUINN: It is fascinating. The patients started on a standard dose of
sorafenib — 400 milligrams twice a day for one month. If they did not
develop particular toxicities, the dose was escalated to 600 milligrams twice a
day and then after another month to 800 milligrams twice a day.
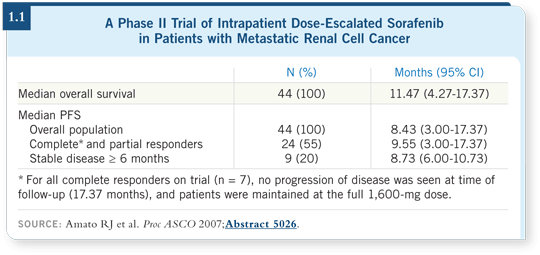
A majority of the patients were able to receive the total dose of 1,600 milligrams a day. Dr Amato reported a decent-sized, albeit Phase II, dose-escalation trial with a complete and partial response rate of 55 percent, which is high (Amato 2007; [1.1]). This study suggests that a proportion of patients need to have an escalation of sorafenib to maximize the response.
In reviewing this, Dr Figlin noted that we haven’t yet seen this with the TKIs. In the TARGET study, which might be considered a standard for sorafenib, the investigator-reported response rate was 10 percent (Escudier 2007b).
Track 14
![]() DR QUINN: It appears that sorafenib has activity in the first-line setting, based
on the report from the ARCCS study (Knox 2007; Ryan 2007). This runs
counter to the randomized Phase II comparison of sorafenib and interferon
suggesting that sorafenib was not so good in the first-line setting (Szczylik
2007). The question is, which set of data do you believe? I believe we have to evaluate it in clinical practice, and my clinical practice in the first-line setting
runs closer to the ARCCS data than to the randomized Phase II study.
DR QUINN: It appears that sorafenib has activity in the first-line setting, based
on the report from the ARCCS study (Knox 2007; Ryan 2007). This runs
counter to the randomized Phase II comparison of sorafenib and interferon
suggesting that sorafenib was not so good in the first-line setting (Szczylik
2007). The question is, which set of data do you believe? I believe we have to evaluate it in clinical practice, and my clinical practice in the first-line setting
runs closer to the ARCCS data than to the randomized Phase II study.
The other presentations from the ARCCS trial were on selected groups of patients. Among the patients with brain metastases that had been previously irradiated or surgically removed, no CNS hemorrhages were recorded and the rates of disease benefit were similar (Henderson 2007). The other big set of data demonstrated activity in nonclear cell types of cancer, particularly papillary and chromophobe subtypes (Stadler 2007).
| Terms of Use/Disclaimer | Privacy Policy | Hardware/Software Requirements Copyright © 2007 Research To Practice. All Rights Reserved. |