
 |
|||||

| Tracks 1-10 | ||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR ESCUDIER: Bevacizumab has previously been shown to be active in kidney
cancer. The first study was presented at ASCO in 2002 (Yang 2002) and
demonstrated that when you administer bevacizumab (10 mg/kg every two
weeks) to patients who have failed high-dose IL-2, you can achieve a significant
improvement in progression-free survival (PFS) compared to placebo.
DR ESCUDIER: Bevacizumab has previously been shown to be active in kidney
cancer. The first study was presented at ASCO in 2002 (Yang 2002) and
demonstrated that when you administer bevacizumab (10 mg/kg every two
weeks) to patients who have failed high-dose IL-2, you can achieve a significant
improvement in progression-free survival (PFS) compared to placebo.
At ASCO 2006, Ron Bukowski presented an interesting study (Bukowski 2006), which aimed to determine whether adding erlotinib to bevacizumab could improve PFS. That trial turned out to be negative, but in the bevacizumab- alone arm, PFS was 8.5 months among previously untreated patients. Together, these observations suggested that bevacizumab was probably an active drug in kidney cancer — that was our hypothesis when we started our trial.
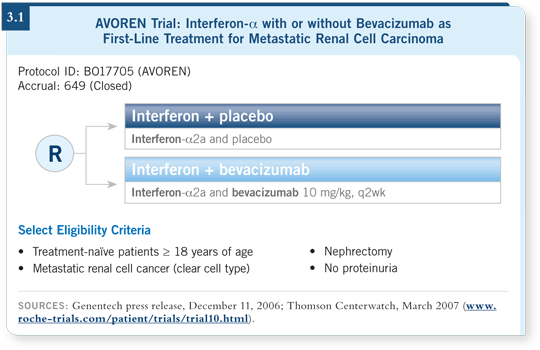
A lot of discussion ensued about what the control arm should be. At the time, interferon was the standard, so we decided to add bevacizumab to interferon, and to avoid any bias, we chose to conduct a double-blind, randomized, controlled study with a placebo arm (3.1).
This is interesting, and it’s different from the CALGB study (CALGB-90206; Rini 2004), which doesn’t have a placebo arm, making that study much more difficult to analyze.
We elected to use an interferon dosage that was utilized by Motzer in his sunitinib study (Motzer 2007) and to add bevacizumab at 10 mg/kg every two weeks. Our initial goal was to show an improvement in overall survival, so that was our primary endpoint.
Our data with interferon show that overall survival should be in the range of 13 months, and our statistical hypothesis was that overall survival would increase from 13 to 17 months. We needed 650 patients to demonstrate this difference with an acceptable hazard ratio.
We opted to perform the final overall survival analysis at 445 deaths and a planned interim analysis at 250 deaths. At that time it was specified that we would also perform a final PFS analysis, and depending on the results, the DSMB would recommend unblinding the study if it was positive.
Tracks 2-3
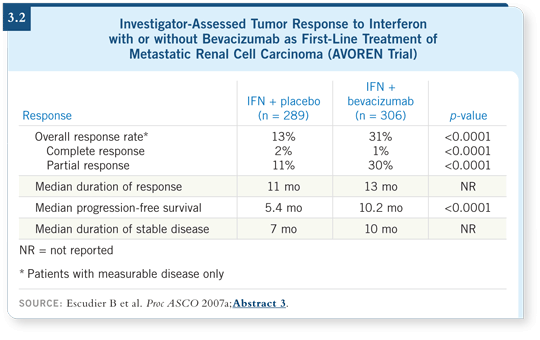
![]() DR ESCUDIER: According to investigator observation, the response rate
increased with the addition of bevacizumab — overall response was 31 percent among those who received interferon/bevacizumab and 13 percent among
those who received interferon/placebo (3.2). The analysis showed a doubling
of PFS, from 5.4 months to 10.2 months.
DR ESCUDIER: According to investigator observation, the response rate
increased with the addition of bevacizumab — overall response was 31 percent among those who received interferon/bevacizumab and 13 percent among
those who received interferon/placebo (3.2). The analysis showed a doubling
of PFS, from 5.4 months to 10.2 months.
When we assessed the different subgroups, we noticed a significant benefit in the favorable risk group, but the benefit was even more important in the intermediate prognostic group, in which the PFS went from 4.5 to 10.2 months (Escudier 2007a).
In the intermediate-risk group, we believe that interferon has little — if any — activity, based on the last study we presented, so we believe that the majority of the effects we have seen in this study are due to the activity of bevacizumab in this group.
![]() DR LOVE: Can you review the safety and tolerability data?
DR LOVE: Can you review the safety and tolerability data?
![]() DR ESCUDIER: More Grade III and IV adverse events were recorded in the
bevacizumab/interferon arm than in the placebo/interferon arm (60 percent
versus 45 percent of the patients, respectively).
DR ESCUDIER: More Grade III and IV adverse events were recorded in the
bevacizumab/interferon arm than in the placebo/interferon arm (60 percent
versus 45 percent of the patients, respectively).
The incidence of fatigue was a little higher in the bevacizumab arm, perhaps due to the additive effects of the drug but perhaps also due to the fact that exposure to interferon was longer in the combination arm.
In terms of bevacizumab-associated side effects, the observed rate of proteinuria was 6.5 percent. The rates of hemorrhage and gastrointestinal perforation were 3.3 and 1.5 percent respectively.
The incidence of death that was not due to progressive disease was two percent in both arms. Of the eight patients in the bevacizumab arm who died, three deaths were possibly related to bevacizumab.
We observed an incidence of 3.9 percent of Grade III hypertension — similar to what we usually see with this type of agent.
![]() DR LOVE: What does this mean for clinical practice? How do you think
people will react to these data in terms of the treatment algorithm for
metastatic disease?
DR LOVE: What does this mean for clinical practice? How do you think
people will react to these data in terms of the treatment algorithm for
metastatic disease?
![]() DR ESCUDIER: Interferon with bevacizumab is now one alternative for
first-line therapy in the metastatic setting. When you view the data with the
AVOREN regimen compared to sunitinib, you see that we are in the same
range of response for PFS (Motzer 2007).
DR ESCUDIER: Interferon with bevacizumab is now one alternative for
first-line therapy in the metastatic setting. When you view the data with the
AVOREN regimen compared to sunitinib, you see that we are in the same
range of response for PFS (Motzer 2007).
In my mind, and perhaps the minds of investigators, side effects are probably a little less troublesome with this regimen than with sunitinib.
The question now is, what is the effect of interferon in this regimen? Some physicians will consider bevacizumab the agent that provides all the benefit. At this point, it’s fair to say we don’t know.
In previous bevacizumab data, PFS was lower than what was recorded with this regimen (Yang 2003; Bukowski 2006), so is interferon necessary to this result? Perhaps it is. Either way, I believe that in Europe we will consider this combination as a standard, and it will compete with sunitinib.
In the United States, my prediction is that many people will consider bevacizumab alone to be enough and that it will compete largely with sunitinib. I would not be surprised if a number of people believe it’s better to start with bevacizumab alone — because it’s easy to administer and has fewer side effects than sunitinib alone — and to keep sunitinib for the second line.
| Terms of Use/Disclaimer | Privacy Policy | Hardware/Software Requirements Copyright © 2007 Research To Practice. All Rights Reserved. |