
 |
|||||

| Tracks 1-20 | ||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR RINI: With an emerging understanding of the biology, it’s clear that the
angiogenic pathways are relevant to kidney cancer pathophysiology, and as
such, several agents have been developed that target various aspects of that
pathway. One class of agents includes small-molecule inhibitors of receptors
that are present on blood vessels and endothelial cells — receptors for vascular
endothelial growth factor, or VEGF.
DR RINI: With an emerging understanding of the biology, it’s clear that the
angiogenic pathways are relevant to kidney cancer pathophysiology, and as
such, several agents have been developed that target various aspects of that
pathway. One class of agents includes small-molecule inhibitors of receptors
that are present on blood vessels and endothelial cells — receptors for vascular
endothelial growth factor, or VEGF.
Agents that block VEGF, such as sunitinib and sorafenib, were both FDA approved in late 2005 or early 2006, because they demonstrated general shrinkage of tumor burden in the majority of patients and objective responses in a smaller subset of patients, ranging anywhere from 10 to up to 40 percent with sunitinib. Sorafenib delayed time to progression in a randomized controlled trial.
Since these FDA approvals, sunitinib was compared to interferon in a frontline study and revealed a fairly dramatic progression-free survival benefit of 11 versus five months and also maintained the response rate of 30 to 40 percent.
Sunitinib has become a front-line reference standard for renal cell cancer. Sorafenib has had less development as initial therapy, and we’re waiting on pending clinical trials for this agent.
Track 2
![]() DR RINI: Bevacizumab has demonstrated tumor shrinkage of around 70
percent and a respectable progression-free survival in the front-line setting.
DR RINI: Bevacizumab has demonstrated tumor shrinkage of around 70
percent and a respectable progression-free survival in the front-line setting.
A large Phase III trial of bevacizumab with interferon versus interferon alone (1.1) should be reported at ASCO this year. I believe it will establish bevacizumab as a standard component of front-line therapy for renal cell cancer.
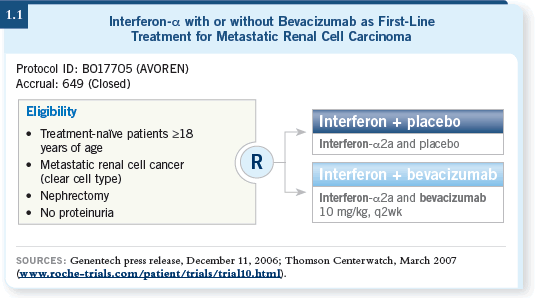
The trial randomly assigned patients to either interferon alone, which was the standard treatment at the time, or the combination of bevacizumab and interferon.
The study was placebo controlled — patients received either interferon/placebo or interferon/bevacizumab. It was powered to evaluate both overall survival and progression-free survival.
According to a press release issued about the trial, the interim analysis showed that bevacizumab significantly prolonged progression-free survival. In addition, this early analysis indicated a trend toward an improvement in overall survival.
Track 4
![]() DR RINI: The predominant side effects of bevacizumab have been, as in other
diseases but perhaps more prominently in renal cancer, hypertension and
proteinuria (Yang 2003). The exact mechanism is not well defined, but these
are common side effects that probably occur in 30 or 40 percent of recipients.
DR RINI: The predominant side effects of bevacizumab have been, as in other
diseases but perhaps more prominently in renal cancer, hypertension and
proteinuria (Yang 2003). The exact mechanism is not well defined, but these
are common side effects that probably occur in 30 or 40 percent of recipients.
These events can be Grade III in five or 10 percent of the patients, depending on the definition. Having said that, bevacizumab is a well-tolerated day-to-day drug.
It’s administered as an IV infusion every other week, and we do not see many common side effects. Hypertension is generally well managed with standard antihypertensive approaches. Proteinuria is not terribly significant clinically unless it reaches the nephrotic range, which is not common.
Bevacizumab carries the risk of the rare but serious side effects of bleeding, clotting, gastrointestinal perforation and other cardiac issues. Those events are relatively uncommon, but again, they are in the rare but serious category.
Track 7
![]() DR RINI: We have no head-to-head comparison, but I believe sorafenib tends
to be a little better tolerated. If you had a group of 100 patients receiving each
drug, it is probably true that sorafenib would be a little better tolerated, on
average. Its side effects generally peak somewhere around weeks four to six,
and then they tend to dissipate.
DR RINI: We have no head-to-head comparison, but I believe sorafenib tends
to be a little better tolerated. If you had a group of 100 patients receiving each
drug, it is probably true that sorafenib would be a little better tolerated, on
average. Its side effects generally peak somewhere around weeks four to six,
and then they tend to dissipate.
Sunitinib is dosed differently. It’s administered intermittently — four weeks on, two weeks off. So those side effects tend to build up over four weeks, resolve over two weeks and then reappear during the dosing period.
The timing, the schedule and the appearance of side effects are different for each agent.
Track 9
![]() DR RINI: We assessed a large number of our patients with metastatic kidney
cancer who received sunitinib on one of a variety of trials. We began
performing thyroid function tests routinely at baseline and then every two
cycles because of reports of thyroid dysfunction associated with sunitinib
treatment in patients with GIST. We found that approximately 85 percent
of patients (56 out of 66) had one or more abnormalities related to thyroid
function.
DR RINI: We assessed a large number of our patients with metastatic kidney
cancer who received sunitinib on one of a variety of trials. We began
performing thyroid function tests routinely at baseline and then every two
cycles because of reports of thyroid dysfunction associated with sunitinib
treatment in patients with GIST. We found that approximately 85 percent
of patients (56 out of 66) had one or more abnormalities related to thyroid
function.
A smaller subset — approximately half of those patients — had more than one abnormality or exhibited clinical signs or symptoms consistent with hypothyroidism. Seventeen patients received thyroid replacement, and about half of them showed improvement in symptoms.

If patients stay on sunitinib therapy long enough, there is evidence that they are likely to develop hypothyroidism. It should be monitored, and if patients develop hypothyroidism, it should be treated.
Track 10
![]() DR RINI: The large Intergroup trial by ECOG started a few months ago and
has accrued a few hundred patients with kidney cancer who have undergone
a nephrectomy (ECOG-E2805; [1.2]). These patients do not have metastatic
disease, but they are at high risk for cancer recurrence based on size, grade,
lymph node involvement and other factors. Eligible patients are randomly
assigned to placebo, sunitinib or sorafenib for one year. It’s a blinded trial, so
all the pills are matched.
DR RINI: The large Intergroup trial by ECOG started a few months ago and
has accrued a few hundred patients with kidney cancer who have undergone
a nephrectomy (ECOG-E2805; [1.2]). These patients do not have metastatic
disease, but they are at high risk for cancer recurrence based on size, grade,
lymph node involvement and other factors. Eligible patients are randomly
assigned to placebo, sunitinib or sorafenib for one year. It’s a blinded trial, so
all the pills are matched.
The primary endpoint is disease-free survival. The target accrual is approximately 1,300 patients, which should take an additional 3 to 3.5 years for enrollment, and then it’ll take some time after that to record recurrences and meet the endpoint.
| Terms of Use and General Disclaimer. | Privacy Policy Copyright © 2007 Research To Practice. All Rights Reserved. |