
 |
|||||

| Tracks 1-17 | ||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR FIGLIN: The paradigm with kidney cancer is evolving. In December 2005, sorafenib was approved for metastatic renal cell cancer. In January 2006,
sunitinib was approved for advanced renal cell cancer. A plenary session at
ASCO 2006 presented data on temsirolimus (Hudes 2006), another agent that
will soon become available.
DR FIGLIN: The paradigm with kidney cancer is evolving. In December 2005, sorafenib was approved for metastatic renal cell cancer. In January 2006,
sunitinib was approved for advanced renal cell cancer. A plenary session at
ASCO 2006 presented data on temsirolimus (Hudes 2006), another agent that
will soon become available.
Let me paint the picture for why these agents have resulted in such spectacular benefit. Clear cell kidney cancer most often has a genetic abnormality associated with the von Hippel-Lindau gene, which activates the hypoxia-inducible factor (HIF), which then activates the vascular endothelial growth factor (VEGF) and angiogenesis pathways.
One could imagine that if we had agents that could inhibit HIF or the angiogenesis pathway, alone or in combination, we would have drugs that may be effective for kidney cancer.
In 2007 and through 2008, we will have at least four drugs that meet that description. We have the VEGF receptor tyrosine kinase inhibitors (TKIs) sorafenib and sunitinib, which inhibit the activation of the VEGF receptor. We have bevacizumab, a VEGF ligand antibody that inhibits before activation of the receptor. We also have temsirolimus, which inhibits HIF and therefore angiogenesis.
![]() DR LOVE: Could you clarify how HIF interacts with VEGF?
DR LOVE: Could you clarify how HIF interacts with VEGF?
![]() DR FIGLIN: Angiogenesis is a downstream effect of HIF — HIF activation
activates angiogenesis and angiogenesis activates the receptor, all in a sequence
of steps. HIF inhibition results in a downregulation of VEGF and other proan-giogenic
factors. Therefore, if you inhibit HIF, you also inhibit angiogenesis.
DR FIGLIN: Angiogenesis is a downstream effect of HIF — HIF activation
activates angiogenesis and angiogenesis activates the receptor, all in a sequence
of steps. HIF inhibition results in a downregulation of VEGF and other proan-giogenic
factors. Therefore, if you inhibit HIF, you also inhibit angiogenesis.
Track 5-6
![]() DR FIGLIN: Sunitinib is a small-molecule oral TKI that, when administered
to patients with good- and intermediate-prognosis renal cell cancer, significantly
improved progression-free survival compared to standard interferon
therapy (Motzer 2007).
DR FIGLIN: Sunitinib is a small-molecule oral TKI that, when administered
to patients with good- and intermediate-prognosis renal cell cancer, significantly
improved progression-free survival compared to standard interferon
therapy (Motzer 2007).
This treatment is associated with a more than twofold improvement in progression-free survival. This improvement occurs across all treatment groups, including patients with good and intermediate prognoses.
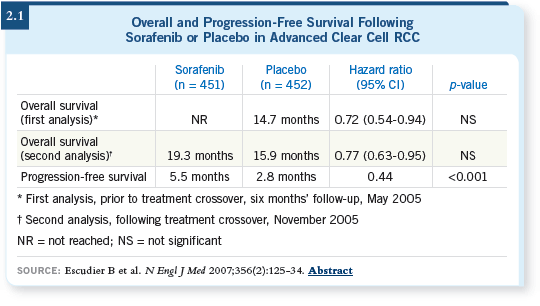
Sorafenib is also a VEGF receptor TKI. It was approved in December 2005 because of a trial demonstrating that sorafenib, when compared to placebo in previously cytokine-treated patients, conferred a significant progression-free survival difference (Escudier 2007; [2.1]).
![]() DR LOVE: What do we know about temsirolimus?
DR LOVE: What do we know about temsirolimus?
![]() DR FIGLIN: Temsirolimus is an ester of rapamycin that inhibits HIF and
is administered intravenously at 25 milligrams weekly. We hope it will be
approved by the FDA soon. Patients tolerate the therapy well.
DR FIGLIN: Temsirolimus is an ester of rapamycin that inhibits HIF and
is administered intravenously at 25 milligrams weekly. We hope it will be
approved by the FDA soon. Patients tolerate the therapy well.
Randomized trials presented last year at ASCO showed a significant improvement in progression-free and overall survival among patients with intermediate- and poor-prognosis renal cell cancer (Hudes 2006).
This has resulted in a dramatic opportunity for patients that heretofore were sent home for hospice care. Temsirolimus is the first treatment that has been shown to produce a survival benefit in renal cell cancer, which is truly no small feat.
Track 8
![]() DR FIGLIN: Bevacizumab was the first drug in kidney cancer that demonstrated
an improvement in progression-free survival (Yang 2003). Although
the time to progression was better, it was unclear exactly how to develop the
drug.
DR FIGLIN: Bevacizumab was the first drug in kidney cancer that demonstrated
an improvement in progression-free survival (Yang 2003). Although
the time to progression was better, it was unclear exactly how to develop the
drug.
A series of trials ensued, the second of which we presented last year at ASCO (Bukowski 2006) that compared bevacizumab and erlotinib to bevacizumab and placebo and showed that approximately 13 to 14 percent of patients had objective responses and another 60 to 65 percent of patients had stable disease with bevacizumab.
However, the addition of erlotinib added no benefit with respect to progression-free survival. It appeared that bevacizumab alone showed substantial activity in untreated patients, with progression-free survival in the eight- to nine-month range.
![]() DR LOVE: What about objective response rates to treatment with bevacizumab
alone?
DR LOVE: What about objective response rates to treatment with bevacizumab
alone?
![]() DR FIGLIN: The objective response rate to bevacizumab alone is approximately 13 to 14 percent among previously untreated patients and about 10
percent among previously treated patients. It is important for the oncologist
not to focus only on the response rate. The overall disease-control rate of
about 80 percent is the significant piece of information.
DR FIGLIN: The objective response rate to bevacizumab alone is approximately 13 to 14 percent among previously untreated patients and about 10
percent among previously treated patients. It is important for the oncologist
not to focus only on the response rate. The overall disease-control rate of
about 80 percent is the significant piece of information.
Currently, two trials evaluating bevacizumab in RCC are taking place, one in the United States (CALGB-90206; Rini 2004) and one in Europe (BO17705; [1.1, page 4]). Each of these trials is a comparison of bevacizumab/interferon to interferon alone.
A recent press release indicated that BO17705 was a statistically positive trial, with an improvement in progression-free survival in the bevacizumab-treated group compared to the control group (Genentech BioOncology 2006). We expect to hear the results in an oral presentation at ASCO 2007.
![]() DR LOVE: What is the side-effect and tolerability profile of the combination?
DR LOVE: What is the side-effect and tolerability profile of the combination?
![]() DR FIGLIN: We don’t have much information yet on the side-effect profile of
bevacizumab and interferon in combination because it’s never been reported
except in Phase I trials.
DR FIGLIN: We don’t have much information yet on the side-effect profile of
bevacizumab and interferon in combination because it’s never been reported
except in Phase I trials.
Bevacizumab, when administered alone, has good tolerability. The major side effects when administered intravenously every two weeks are basically hypertension and proteinuria, which could lead to nephrotic syndrome.
Bevacizumab doesn’t produce the lethargy, anorexia, hand-foot syndrome and other immunosuppressive complications associated with TKIs. We need to consider carefully what happens when interferon is added because interferon does involve toxicities when administered three times a week.
Track 9
![]() DR FIGLIN: We have a clear suggestion (Atkins 2004) that patients with high-risk
kidney cancer have a disease that is more often driven by a pathway called
mammalian target of rapamycin (mTOR). Temsirolimus is an mTOR inhibitor
that inhibits HIF and may be more appropriate for the patient at intermediate
or poor risk.
DR FIGLIN: We have a clear suggestion (Atkins 2004) that patients with high-risk
kidney cancer have a disease that is more often driven by a pathway called
mammalian target of rapamycin (mTOR). Temsirolimus is an mTOR inhibitor
that inhibits HIF and may be more appropriate for the patient at intermediate
or poor risk.
Patients with good and intermediate prognoses may have a more VEGF-driven pathway, and it may be more appropriate to use targeted agents against that specific pathway.
As we better understand the biology of this cancer, we can begin to tailor the multiple treatments to that biology, as opposed to just administering the drugs to all comers. One of the challenges I have as a translational clinical investigator is to help define for practicing oncologists when to use sunitinib, when to use temsirolimus and when neither agent should be used.
Track 13
![]() DR FIGLIN: The most significant dose-limiting toxicity is hand-foot
syndrome. It occurs in approximately 20 to 30 percent of patients.
DR FIGLIN: The most significant dose-limiting toxicity is hand-foot
syndrome. It occurs in approximately 20 to 30 percent of patients.
Even Grade I and Grade II hand-foot syndrome can be troublesome for patients. It can affect their ability to walk normally and to carry out normal activities. We must be aware of this early in the course of treatment.
The biology of hand-foot syndrome is not well understood. It’s not clear whether it’s the inhibition of angiogenesis or “off-target” effects of multitargeted agents, but if you intervene early, hand-foot syndrome is rapidly reversible.
| Terms of Use and General Disclaimer. | Privacy Policy Copyright © 2007 Research To Practice. All Rights Reserved. |