
 |
|||||

| Tracks 1-20 | ||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 3
![]() DR ATKINS: Renal cell cancer is unique in that it appears to be the one solid
tumor that is directly sensitive to anti-angiogenic or anti-VEGF pathway
inhibitors when used as single agents. In other cancers, drugs like bevacizumab
have shown activity but all in combination with chemotherapy. That
brings into play a lot of other mechanisms.
DR ATKINS: Renal cell cancer is unique in that it appears to be the one solid
tumor that is directly sensitive to anti-angiogenic or anti-VEGF pathway
inhibitors when used as single agents. In other cancers, drugs like bevacizumab
have shown activity but all in combination with chemotherapy. That
brings into play a lot of other mechanisms.
When we talk about renal cell cancer being sensitive to these agents as single agents, we believe the reason is that kidney cancer cells have “grown up” surrounded by VEGF and haven’t had to work to develop other means of obtaining a blood supply.
This tumor is highly dependent on VEGF, and when you administer a drug like bevacizumab that binds VEGF or a drug that blocks the receptors, like sunitinib or sorafenib, you see almost immediate effects. That’s a unique situation directly related to the biology of kidney cancer.
What are these drugs? One of the two that have been approved in the past year and a half is sorafenib, which is an oral multikinase inhibitor that inhibits Raf within tumor cells and the Raf kinase pathway and the VEGF-R2 receptor within the endothelial cells (Brugarolas 2007; [3.1]).
These two drugs, when administered to patients with advanced renal cell cancer, cause tumor shrinkage in 60 to 80 percent of patients and delay time to progression relative to control therapy.
It’s important to note that a lot of different receptors have tyrosine kinases on them, so these agents are “dirty drugs.” They not only inhibit VEGF-R2, which we believe is responsible for most of their activity, but they also inhibit other kinases. This may contribute to their activity or to their toxicity or may even produce countervailing effects that inhibit their activity.
Track 8
![]() DR ATKINS: Three approaches to combination therapy have been explored
in renal cell cancer. One is trying to combine targeted agents — whether
targeted against a tumor or the blood vessel — with immunotherapy. Another
is combining targeted agents vertically — hitting a particular pathway at two
different sites — such as binding the ligand and inhibiting the receptor simultaneously.
The third approach is what we call a horizontal blockade, by which
you inhibit two different pathways or parallel pathways.
DR ATKINS: Three approaches to combination therapy have been explored
in renal cell cancer. One is trying to combine targeted agents — whether
targeted against a tumor or the blood vessel — with immunotherapy. Another
is combining targeted agents vertically — hitting a particular pathway at two
different sites — such as binding the ligand and inhibiting the receptor simultaneously.
The third approach is what we call a horizontal blockade, by which
you inhibit two different pathways or parallel pathways.
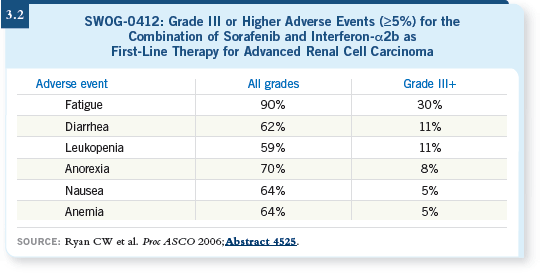
Studies evaluating combinations of targeted agents with immunotherapy include one presented at ASCO last year (Ryan 2006) that evaluated sorafenib in combination with interferon, which will be published shortly. They presented encouraging results, such as 30 percent response rates and no more toxicity than one would expect, and it seems that you can combine those two agents without negative consequences (3.2).
At least in those studies, five or 10 percent of patients showed complete responses, so you might obtain the complete and durable response benefit of immunotherapy with the tumor shrinkage benefits of VEGF receptor therapy.
We will see a similar type of data at ASCO this year on bevacizumab with interferon, which is another approach to combining a targeted agent with immunotherapy, and our group is actively investigating combinations of bevacizumab with high-dose interleukin-2.
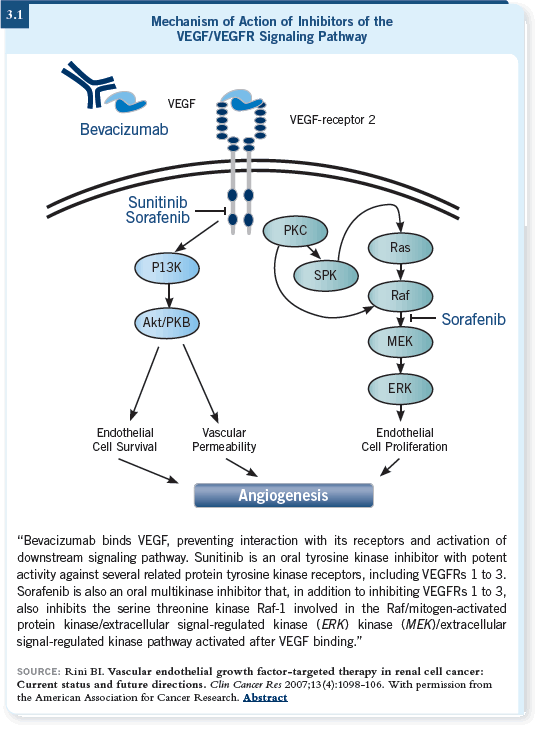
We’ve spent a lot of time evaluating the vertical inhibition of the VEGF pathway. The theory behind this approach is that when you block the VEGF receptor, you make the cells “hypoxic” and cause increases in circulating VEGF. This could potentially drive angiogenesis if the VEGF receptor isn’t completely blocked, so binding the circulating VEGF may create a better block on the pathway.
We found that when you combine sorafenib and bevacizumab, you see more potent activity than with either agent alone. Synergistic activity is seen in close to 50 percent of patients, but the toxicity is also synergistic. We see much more toxicity than with either agent alone and have had to reduce the dose of each agent significantly to find a tolerable dose.
The more promising approach is the horizontal blockade with combination therapy, by which you might inhibit two pathways at the same time.
For example, promising results are being obtained by inhibiting the mTOR pathway and the VEGF pathway together. We’re all interested in exploring that further in Phase II trials. Studies have also been launched evaluating sorafenib with temsirolimus.
Track 10
![]() DR ATKINS: We know very little. Anecdotal and observational studies are
emerging. However, a formal study indicated that sunitinib has activity in
patients whose disease progressed after bevacizumab (Rini 2006). You may
see tumor shrinkage in close to the same number of patients, although not as
robust and for not as long.
DR ATKINS: We know very little. Anecdotal and observational studies are
emerging. However, a formal study indicated that sunitinib has activity in
patients whose disease progressed after bevacizumab (Rini 2006). You may
see tumor shrinkage in close to the same number of patients, although not as
robust and for not as long.
It’s also beginning to appear that if you take a break and restart the same agent, possibly at a higher dose, you can see a little response again. The physiologic resistance mechanism is plastic in some regards in that if you give a patient a break from that particular agent, you might be able to obtain a benefit again.
I believe we’ll see activity in sorafenib after sunitinib failures and sunitinib after sorafenib failures. The real question is, is the response more than you would see if you just put them back on the same agent again?
The greatest potential for seeing sequential activity right now lies in studying mTOR inhibitors in tumors that have become refractory to sunitinib or sorafenib, and those studies are ongoing — big, industry-sponsored trials that will evaluate mTOR inhibition versus placebo or addition of an mTOR inhibitor versus switching to an mTOR inhibitor in patients whose disease progresses on a VEGF receptor TKI.
Track 13
![]() DR ATKINS: I believe sunitinib produces more fatigue, more problems with
blood count and more problems with diarrhea and has also been shown to
produce hypothyroidism. Also, in a small number of patients, it results in
cardiac effects, including decreases in ejection fraction.
DR ATKINS: I believe sunitinib produces more fatigue, more problems with
blood count and more problems with diarrhea and has also been shown to
produce hypothyroidism. Also, in a small number of patients, it results in
cardiac effects, including decreases in ejection fraction.
Sorafenib is more likely to produce rash and hand-foot syndrome and less likely to produce fatigue, although no formal comparison has been made between the two drugs from a toxicity standpoint. The adjuvant trial that involves sorafenib, sunitinib and a placebo will be a good opportunity to observe the differences in toxicity in patients who don’t have disease-related symptoms.
| Terms of Use and General Disclaimer. | Privacy Policy Copyright © 2007 Research To Practice. All Rights Reserved. |