
 |
|||||

Tracks 1-11 |
||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 8
![]() DR DUTCHER: This study had an interesting design. The concept was that
these drugs would not necessarily produce complete or partial responses, but
they would delay tumor growth, and therefore we would see delay in progression.
DR DUTCHER: This study had an interesting design. The concept was that
these drugs would not necessarily produce complete or partial responses, but
they would delay tumor growth, and therefore we would see delay in progression.
This design is probably more acceptable in renal cell treatment than it is for other tumors. Obviously, survival is the ultimate endpoint, but the surrogate is progression-free survival. If we’re not going to see responses, then we want to determine whether the drug will affect the natural history of the disease.
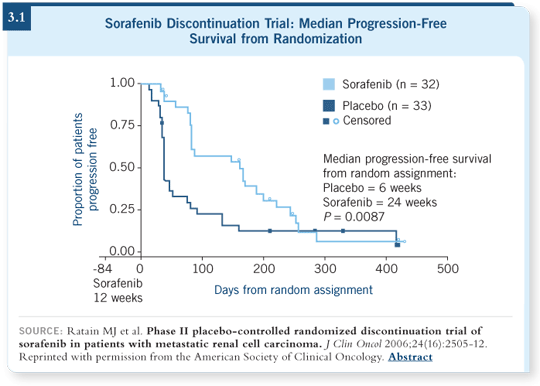
In the randomized discontinuation trial, all the patients received sorafenib for 12 weeks. Then, if they experienced 25 percent shrinkage as measured by RECIST, that was seen as some evidence of response, and they continued on the treatment. If they had 25 percent growth, they were taken off the treatment.
If at 12 weeks they were somewhere in the middle — no growth, no shrinkage — they were randomly assigned to a placebo or to continue sorafenib for another 12 weeks. At the end of 24 weeks, the number of patients on sorafenib who had not progressed compared to the placebo had doubled (Ratain 2006; [3.1]).
The randomized discontinuation trial demonstrated that sorafenib had some effect and continuing it had some effect and that when sorafenib was stopped, growth of the tumor continued. It showed ability to inhibit progression, so that was the basis for the randomized placebo-controlled study known as the TARGETs trial.
![]() DR LOVE: What did the TARGETs trial show?
DR LOVE: What did the TARGETs trial show?
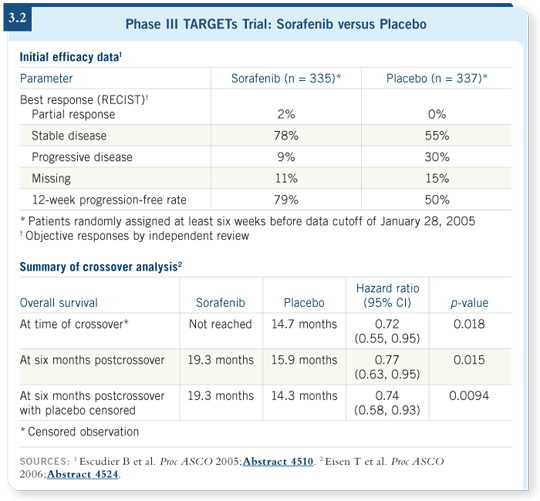
![]() DR DUTCHER: This was an international study of sorafenib versus placebo for
patients who had received one prior systemic therapy for advanced renal cell
carcinoma. There was a significant improvement in progression-free survival
for the patients on sorafenib (Escudier 2005; [3.2]).
DR DUTCHER: This was an international study of sorafenib versus placebo for
patients who had received one prior systemic therapy for advanced renal cell
carcinoma. There was a significant improvement in progression-free survival
for the patients on sorafenib (Escudier 2005; [3.2]).
The difficulty with this study is that once the progression-free survival was observed, it was felt to be unethical to continue the trial with a placebo. The patients were unblinded, and patients receiving the placebo were allowed to receive sorafenib; that may confound the survival data.
Data presented at ASCO in 2006 demonstrated that patients who crossed over received benefit from starting sorafenib, even after having progressed on the placebo (Eisen 2006). It’s pretty clear that we will see continued benefit in both arms of that study.
| Terms of Use and General Disclaimer| Privacy Policy Copyright © 2008 Research To Practice. All Rights Reserved. |