
 |
|||||

Tracks 1-15 |
||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 6
![]() DR BUKOWSKI: I believe the two drugs would come out pretty close. One
of the issues revolves around the side-effect profiles and which drug is easier
to tolerate, and the other issue is which drug is more powerful in terms of its
effect on the surrogates of clinical benefit.
DR BUKOWSKI: I believe the two drugs would come out pretty close. One
of the issues revolves around the side-effect profiles and which drug is easier
to tolerate, and the other issue is which drug is more powerful in terms of its
effect on the surrogates of clinical benefit.
The surrogates are delaying progression of the cancer or improving survival, because response — although it’s important — is not the endpoint we focus on, unless patients are symptomatic, which is not the case for the vast majority.
When you look at the two drugs, you see that the major response rates by RECIST (Response Evaluation Criteria in Solid Tumors) are different — higher with sunitinib than with sorafenib (Motzer 2006b; Escudier 2005). The numbers of patients who actually have a decrease in their tumor size are similar at about 70 to 75 percent. Overall, the same number of patients benefit. The magnitude may be slightly different in terms of response, but response doesn’t necessarily control the progression times or survival.
Track 7
![]() DR BUKOWSKI: We have few data for sequencing, but that’s probably the way
these agents will be used. Our group presented data on a series of patients who
received treatment with one multikinase inhibitor (MKI), such as sunitinib or
sorafenib, and then were placed on the other after their disease progressed. We
found a series of patients who clearly responded to the second MKI (Tamaskar
2006).
DR BUKOWSKI: We have few data for sequencing, but that’s probably the way
these agents will be used. Our group presented data on a series of patients who
received treatment with one multikinase inhibitor (MKI), such as sunitinib or
sorafenib, and then were placed on the other after their disease progressed. We
found a series of patients who clearly responded to the second MKI (Tamaskar
2006).
When doctors in the community begin to treat patients with these drugs, they have to understand that no specific duration of treatment is recommended. Ordinarily, we use disease progression as the indicator to stop a drug or start a new drug. Here, although disease progression is looked for, we don’t necessarily use that as an indicator to stop or to change treatment.
We have continued — in many of the clinical trials that have been presented — with the same MKI in the face of progressive disease should the patient not have new or worse symptoms. The assumption is that continued inhibition of the VEGF pathway is the important part, and if you take away that inhibition quickly without providing another way to inhibit that pathway, whether it’s bevacizumab or another kinase inhibitor, you could have deleterious effects.
Tracks 9, 11
![]() DR BUKOWSKI: They are different, and the differences probably relate to the
kinases they inhibit. Kinases are ubiquitous to all cells in the body. Hence,
you might expect some of these drugs to have unsuspected toxicities. With
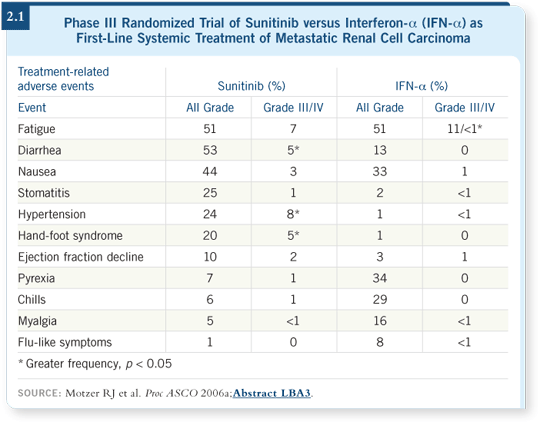
sunitinib, the predominant toxicity is fatigue. Interferon has the same, if not
greater, toxicity in terms of fatigue (Motzer 2006a; [2.1]), but the fatigue is
sometimes limiting in terms of the dose.
DR BUKOWSKI: They are different, and the differences probably relate to the
kinases they inhibit. Kinases are ubiquitous to all cells in the body. Hence,
you might expect some of these drugs to have unsuspected toxicities. With
sunitinib, the predominant toxicity is fatigue. Interferon has the same, if not
greater, toxicity in terms of fatigue (Motzer 2006a; [2.1]), but the fatigue is
sometimes limiting in terms of the dose.
If you use 50 mg/day of sunitinib continuously, fatigue becomes overriding. Patients can’t tolerate that dose continuously. The longer they receive the drug, the longer it takes for them to recover. Sometimes patients need up to three weeks to make a full recovery from the fatigue. The alternative is to lower the dose to 37.5 mg/day, which is the second dose level for sunitinib.
![]() DR BUKOWSKI: Sorafenib is an easier drug to use than sunitinib; it has fewer
side effects. Its two main side effects are skin toxicity and diarrhea, occurring
in about 30 percent of patients (Escudier 2005). The diarrhea is not a problem;
you can control it with diphenoxylate/atropine or loperamide.
DR BUKOWSKI: Sorafenib is an easier drug to use than sunitinib; it has fewer
side effects. Its two main side effects are skin toxicity and diarrhea, occurring
in about 30 percent of patients (Escudier 2005). The diarrhea is not a problem;
you can control it with diphenoxylate/atropine or loperamide.
The skin toxicity is generally what has required dose reductions. It’s hand-foot syndrome, as with the fluoropyrimidines. These patients develop some redness and tenderness. I believe dose modifications because of hand-foot syndrome are required in one third or more of patients.
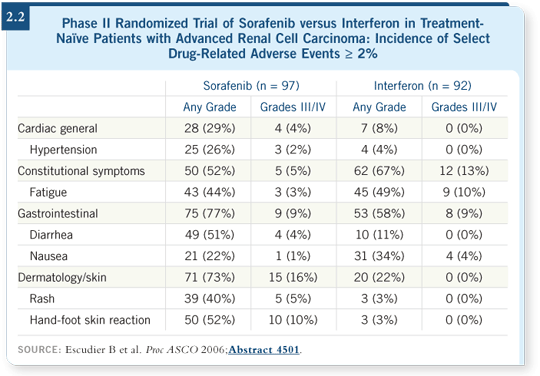
In the study presentation by Dr Escudier at ASCO 2006, in which he presented the toxicity data for previously untreated patients, about 50 percent experienced hand-foot symptoms of any grade (Escudier 2006; [2.2]).
Track 13
![]() DR BUKOWSKI: Temsirolimus is an interesting drug with a different target.
It inhibits a kinase called mammalian target of rapamycin (mTOR). The
study presented at ASCO 2006 was a Phase III trial for patients with renal cell
cancer. The investigators didn’t select for patients with clear cell carcinoma,
but they did select patients who would be expected to have poor-prognosis
metastatic disease. The expected survival of the group was four to five months.
DR BUKOWSKI: Temsirolimus is an interesting drug with a different target.
It inhibits a kinase called mammalian target of rapamycin (mTOR). The
study presented at ASCO 2006 was a Phase III trial for patients with renal cell
cancer. The investigators didn’t select for patients with clear cell carcinoma,
but they did select patients who would be expected to have poor-prognosis
metastatic disease. The expected survival of the group was four to five months.
Is there any reason to suggest that sunitinib or sorafenib cannot be administered to this type of patient? No. I believe you can use those agents for these patients, and you’ll probably have the same effects.
Temsirolimus, however, has been tested only in that group of patients. It did produce an improvement in survival compared to interferon (Hudes 2006). The group receiving temsirolimus at 25 mg once a week had a median survival of about 10.9 months. It was a three-month advantage in median survival, but it was significant. It’s a drug that clearly has an effect. We have to learn how to use it and determine its place in the treatment of this disease.
Track 14
![]() DR BUKOWSKI: For the untreated patient with clear cell carcinoma, you have
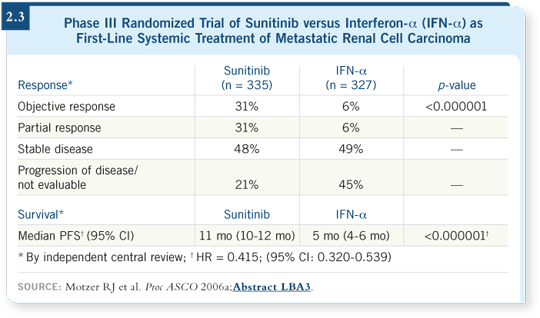
two choices: sunitinib or sorafenib. Probably, given the data presented (Motzer
2006a; [2.3]), sunitinib will be used. It was the featured drug at the ASCO
2006 Plenary Session. I have no doubt that will influence the vast majority of
medical oncologists because the data are pretty solid with the drug.
DR BUKOWSKI: For the untreated patient with clear cell carcinoma, you have
two choices: sunitinib or sorafenib. Probably, given the data presented (Motzer
2006a; [2.3]), sunitinib will be used. It was the featured drug at the ASCO
2006 Plenary Session. I have no doubt that will influence the vast majority of
medical oncologists because the data are pretty solid with the drug.
When those patients progress — and all of them do — you should consider the second kinase inhibitor, sorafenib, as a drug to follow up in the sequential therapy for this disease. If you’re convinced that a subset of patients respond badly and have rapidly progressive disease, temsirolimus is a drug you can use in that situation.
![]() DR LOVE: Are there any patients in whom you’d initiate sorafenib rather than
sunitinib?
DR LOVE: Are there any patients in whom you’d initiate sorafenib rather than
sunitinib?
![]() DR BUKOWSKI: There are patients in whom you can use sorafenib initially
— those who have minimal symptoms and in whom you want to avoid the
side effects associated with sunitinib.
DR BUKOWSKI: There are patients in whom you can use sorafenib initially
— those who have minimal symptoms and in whom you want to avoid the
side effects associated with sunitinib.
I believe the medical oncology community will start to use both of these drugs, and they will decide for themselves which of the two is the most favorable in terms of side effects. They probably have fairly similar effects on the biology of the disease.
Track 15
![]() DR BUKOWSKI: One important study in the United States will compare
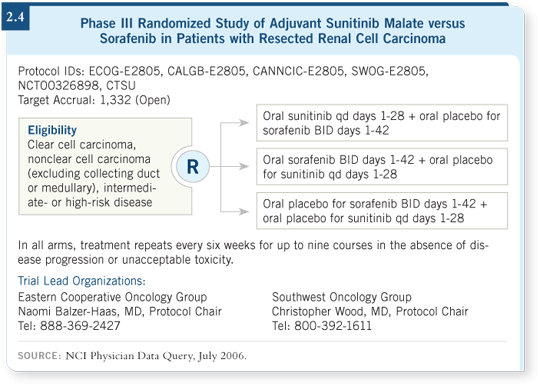
sunitinib, sorafenib and placebo (ECOG-E2805; [2.4]) as adjuvant therapy.
The investigators plan to administer these agents for a year. I believe that
it will be difficult to treat the vast majority of patients for one year with
sunitinib; it may be a little easier with sorafenib.
DR BUKOWSKI: One important study in the United States will compare
sunitinib, sorafenib and placebo (ECOG-E2805; [2.4]) as adjuvant therapy.
The investigators plan to administer these agents for a year. I believe that
it will be difficult to treat the vast majority of patients for one year with
sunitinib; it may be a little easier with sorafenib.
![]() DR LOVE: What do you think will be the key issues with each of these drugs
in administering them for a year?
DR LOVE: What do you think will be the key issues with each of these drugs
in administering them for a year?
![]() DR BUKOWSKI: With sunitinib, I believe it will be fatigue. I don’t know that
patients will tolerate this drug because of the fatigue, and it will require dose
reductions. With sorafenib, it will be skin toxicity. Remember, sorafenib is
administered continuously. These drugs should be administered continuously
because they don’t cure the disease; they suppress it.
DR BUKOWSKI: With sunitinib, I believe it will be fatigue. I don’t know that
patients will tolerate this drug because of the fatigue, and it will require dose
reductions. With sorafenib, it will be skin toxicity. Remember, sorafenib is
administered continuously. These drugs should be administered continuously
because they don’t cure the disease; they suppress it.
Sunitinib is administered on a four-week-on, two-week-off schedule, which is less than optimal. The two-week-off period was developed to let patients recover from the toxicities. During those two weeks, however, some patients’ symptoms start to recur, and the disease may start to progress.
| Terms of Use and General Disclaimer| Privacy Policy Copyright © 2008 Research To Practice. All Rights Reserved. |